Orbital reconstruction
1. Emergency treatment
Emergency treatment in orbital fractures is always indicated in the following situations:
- Partial or complete visual loss due to direct or indirect optic nerve trauma
- Severely increased intraocular pressure
- Acute space-occupying lesion creating increased intraorbital pressure (eg, retrobulbar hematoma, orbital emphysema)
- A severe shift of orbital content
- Severe entrapment of eye muscle (particularly in pediatric patients, “trapdoor”)
Globe rupture and intraocular trauma
These injuries require ophthalmological intervention.
Retrobulbar hematoma
A pressure increase in the periorbital region due to a retrobulbar hematoma can cause significant injury such as the creation of compartment syndrome with injury of the neurovascular structures and the possibility of vision loss.
Read more details about retrobulbar hemorrhage here.
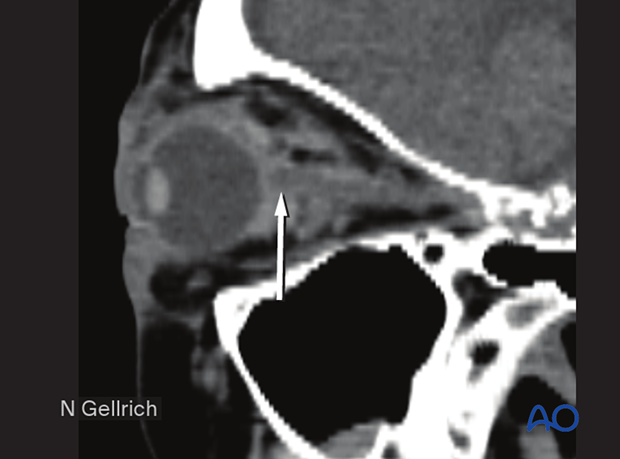
This CT scan sagittal view shows a small retrobulbar hematoma.
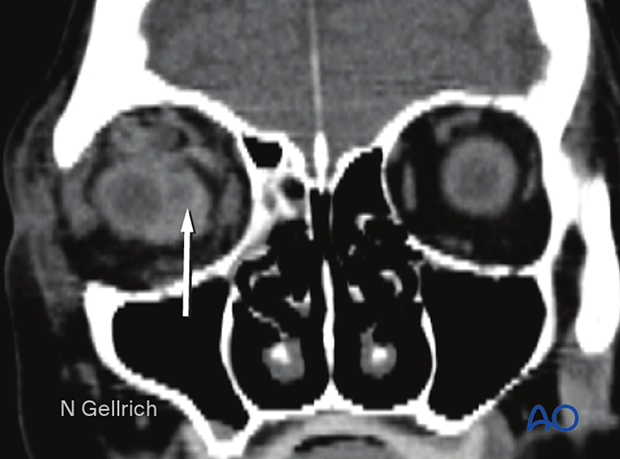
Coronal view of the same retrobulbar hematoma.
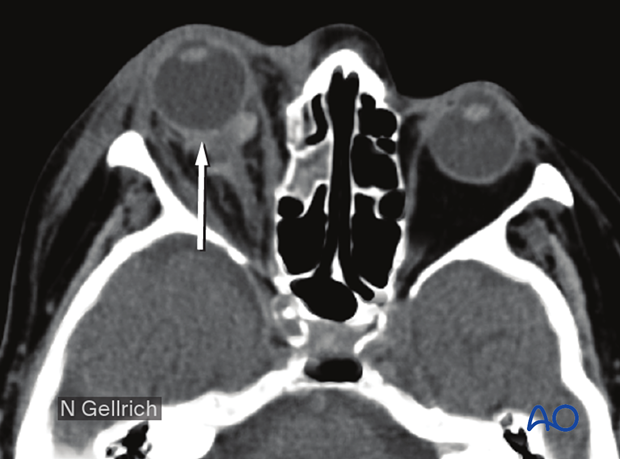
Axial view of the same retrobulbar hematoma.
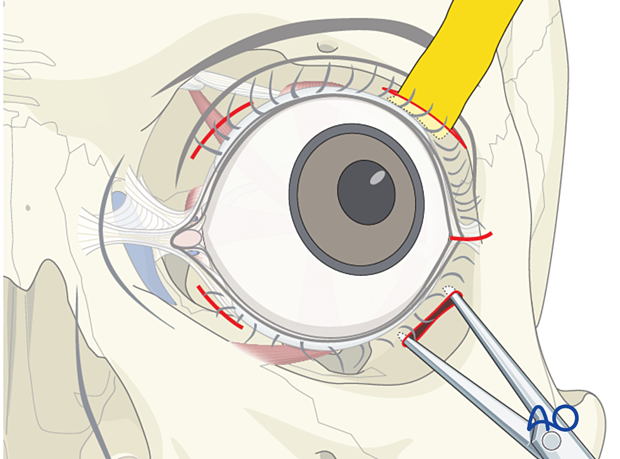
If a retrobulbar hematoma leads to a tense, proptotic globe with acute visual disturbances, emergency decompression should be initiated. This situation requires urgent decompression, such as cantholysis, removal of implanted reconstructive material, and/or evacuation of a loculated hematoma under general or local anesthesia.
Transcutaneous transseptal incisions help evacuate the hematoma and relieve excess periorbital pressure. Alternative methods such as transconjunctival pressure release, lateral canthotomy, and inferior cantholysis may also be considered according to patient condition.
An exception may be pulsatile exophthalmos which can be a sign of a carotid-cavernous sinus fistula. A fistula of this nature requires appropriate preoperative imaging and planning.
Emphysema
Severe emphysema might significantly raise intraorbital pressure. If this compromises visual function or endangers the orbital contents, orbital decompression must be considered.
Patients with signs of intraorbital emphysema are given antibiotics and decongestive nasal drops.
To avoid additional emphysema due to acute pressure rise, patients with sinus fractures in the periorbital region should not blow their nose. They should also be instructed to sneeze with an open mouth to minimize the increase of intranasal/intrasinus pressure.
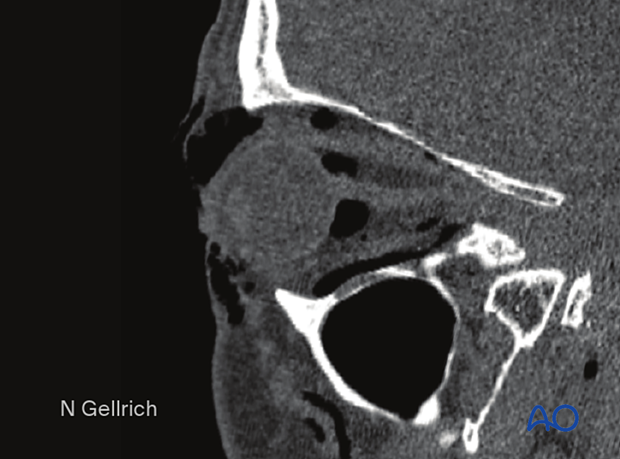
Usually, there is no need for emergency treatment of orbital floor/medial wall fractures unless there is severe ongoing hemorrhage in the orbital cavity, the paranasal sinuses, the nasal cavity, or fractures which create muscle ischemia.
In younger patients there is a danger of necrosis of the entrapped rectus muscle due to the so-called “trapdoor” phenomenon. In such cases, immediate release of entrapped tissues is necessary.
More information on the trapdoor phenomenon can be found in the orbital reconstruction treatment for orbital floor fractures in the Surgery Reference pediatric trauma section.
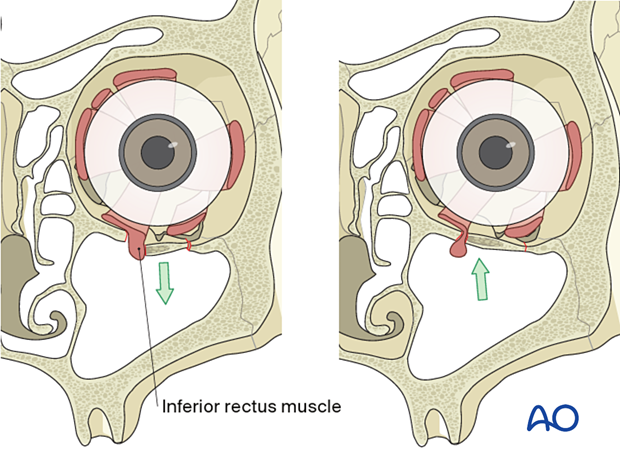
Bone fragments affecting the optic nerve
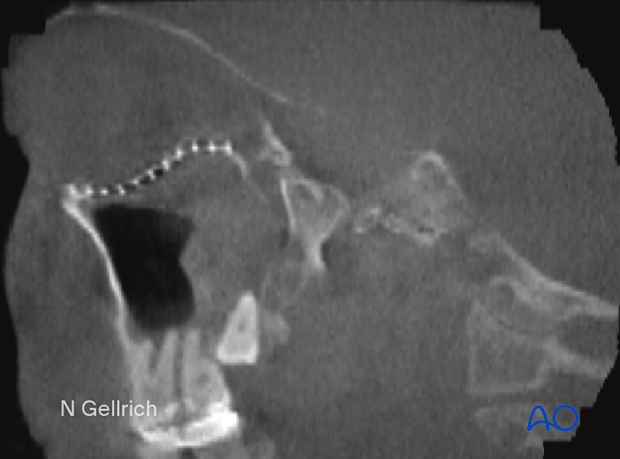
Special attention should be given to the posterior third of the orbit, the superior orbital fissure, and the optic canal. Fractures and hematoma formation in these anatomical areas can be associated with superior orbital fissure syndrome and optic nerve injury.
In this image the axial CT scan in the plane of the optic nerve shows multiple fractures of the lateral orbital wall and the greater wing of the sphenoid of the right side. Compression or displacement (stretching) of the optic nerve may be produced.
Displaced fracture fragments in the posterior third of the orbit may create nerve compression.
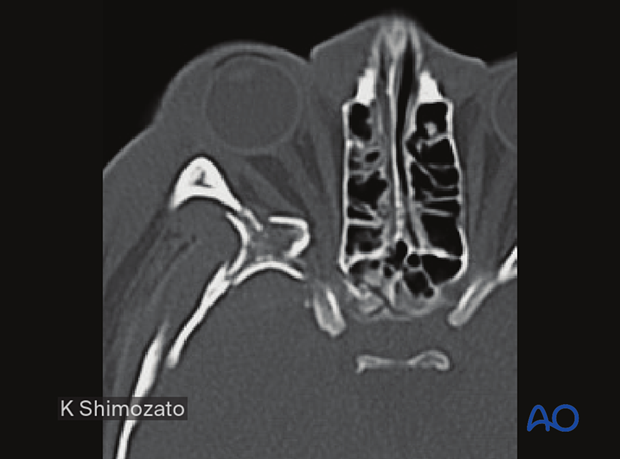
Severe bleeding
In case of severe nasal, oral, or pharyngeal hemorrhage, the following options should be considered:
- Assessment of current medical treatment for anticoagulation such as Coumadin, aspirin, or other antiplatelet medication
- Compression, either by nasal packing, balloon tamponade, or direct compression
- Electrocautery or ligation if a clear bleeding source can be identified
- Normalization of blood pressure
- Interventional radiologic embolization if simpler methods fail
- In some cases, reduction of fracture displacement may significantly reduce bleeding
2. General considerations
Teaching videos
AO Teaching video on fixation of a complex midface fracture
$name
AO Teaching video on fixation of a zygomaticomaxillary fracture and an orbital floor fracture
Visualization of fracture
Appropriate exposure of the orbit requires adequate visualization. Wide exposure of the fracture and adequate lighting is essential when addressing these fractures. Consider using headlights for all orbital surgery.
Special malleable orbital retractors (straight or anatomically formed) are available with metric markings that provide the surgeon with additional information regarding the extent of the fracture and the depth of the orbital dissection. Specific orbital retractors have been developed to improve orbital retraction and minimize soft-tissue prolapse during the insertion of implants.
Unique orbital contours
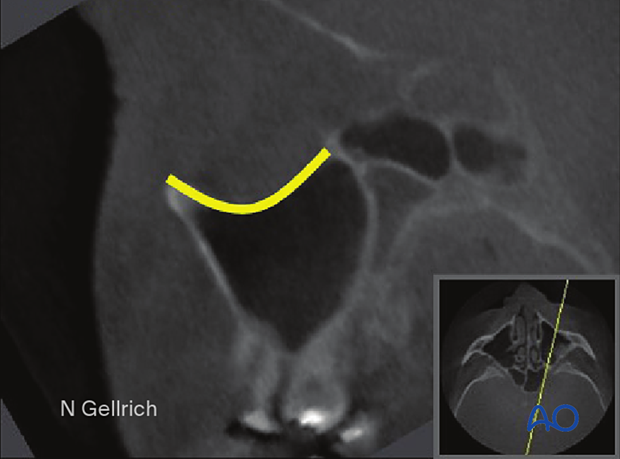
It is crucial to consider the unique undulating contours of the internal orbital cavity.
The key areas of orbital volume constriction are the posterior orbital floor and the medial orbital wall, which constrict orbital volume. This results in a lazy “S-shape” of the orbital floor when viewed from a projection of the floor in the longitudinal axis of the optic nerve. Before the constriction in the posterior third, the contour of the orbital floor slopes inferiorly behind the orbital rim before ascending towards the posterior constriction. These contours must be incorporated into the reconstructive technique to ensure the appropriate soft-tissue volume and shape, recreating the normal appearance of the eye.
Inadequate reconstruction of the undulated contours of the orbital floor commonly leads to a “hammock-shaped” contour. This increases orbital volume and decreases globe projection. This error occurs in cases where bioresorbable implants are used, when the implant is unstable and falls into the maxillary sinus, or when the proper shape and contours of the implant have not been achieved.
The image depicts a small implant displaced into the maxillary sinus postoperatively, increasing the orbital volume.
Transition zone (medial wall/floor)
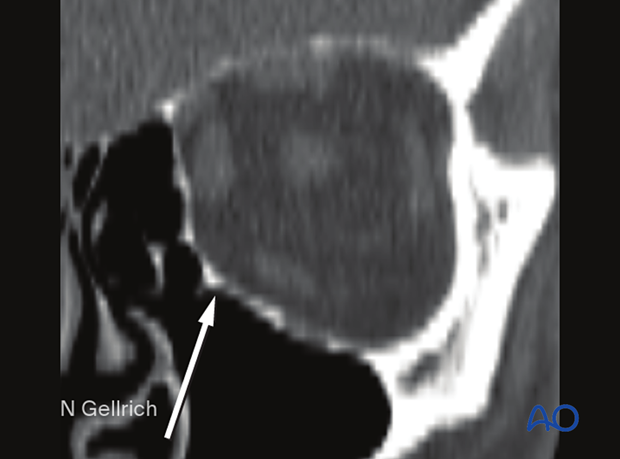
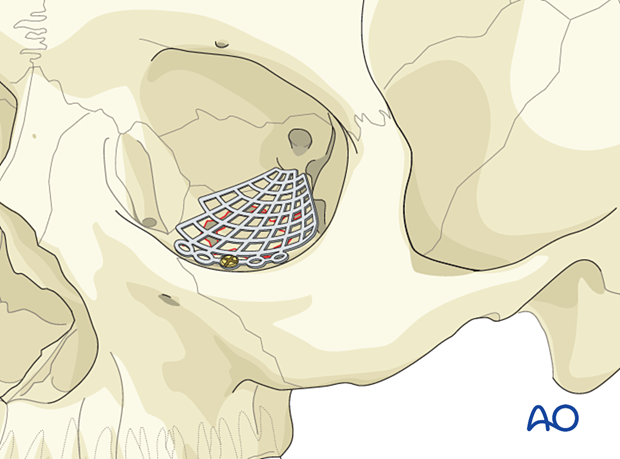
Achieving the proper contours of the orbit is just as important as achieving the proper volume, as these contours create subtle changes in the appearance of the eyes and lids. Repair of the transition zone between the medial orbital wall and floor is vital for determining the position and prominence of the orbital contents and the globe.
Another challenge in orbital repair is recreation of the “inferomedial orbital bulge” where the floor meets the medial orbital wall. Here the volume of the orbit is moderately constricted and contributes to globe prominence.
Coronal slice of a postoperative CT scan taken after repair of the left medial orbital wall and orbital floor.
The red line in the medial wall and orbital floor area indicates the preoperative virtual planning superimposed on the mesh used to reconstruct the area.
In this case, the uninjured contralateral orbit was mirrored during the preoperative planning to help plan the reconstruction of the fractured left orbit. It can also serve as a template to guide the contouring of the orbital implant to accurately restore the complex transition zone between the medial orbit and the floor.
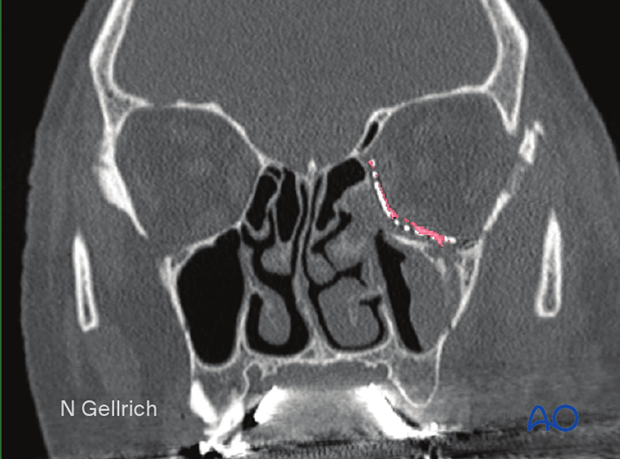
Infraorbital nerve
Any reconstruction of the orbital floor must consider the course of the infraorbital nerve in the orbital floor. The nerve ascends through the infraorbital foramen and travels upward to reach the anterior orbital floor where it is initially in a groove oriented slightly upwards and laterally. After traveling through this initial groove, it enters the canal and continues to travel laterally and posteriorly until it crosses the posterior portion of the inferior orbital fissure where it enters the sphenopalatine fossa.
While decompression might be considered in cases of persistent infraorbital nerve anesthesia, the success of this maneuver has never been documented. Usually, patients with hypesthesia or anesthesia improve substantially over a 6- month period post injury. Most significant hypesthesia is caused by medial impaction of a fractured zygoma and generally responds well to decompression at the time of fracture reduction.
If there is significant compression or dislocation of bone compressing the course of the infraorbital nerve, decompression at the time of exploration should be a routine part of the intraoperative examination after the initial reduction under direct vision. The best results occur when the nerve is assessed and decompressed shortly after the initial injury.
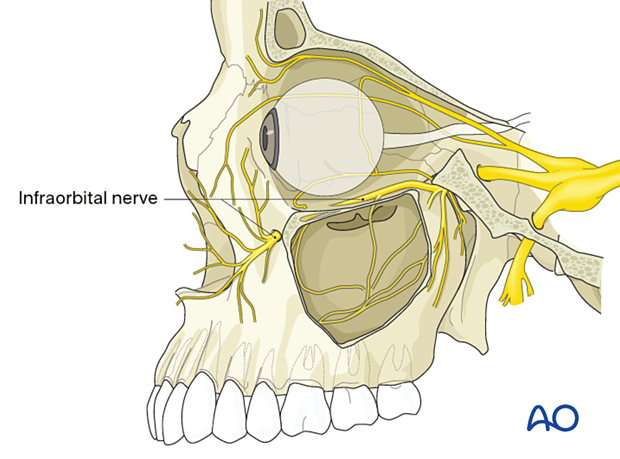
In the pediatric patient, the infraorbital nerve might exit closer to the infraorbital rim. The younger the patient, the closer the nerve exits to the rim.
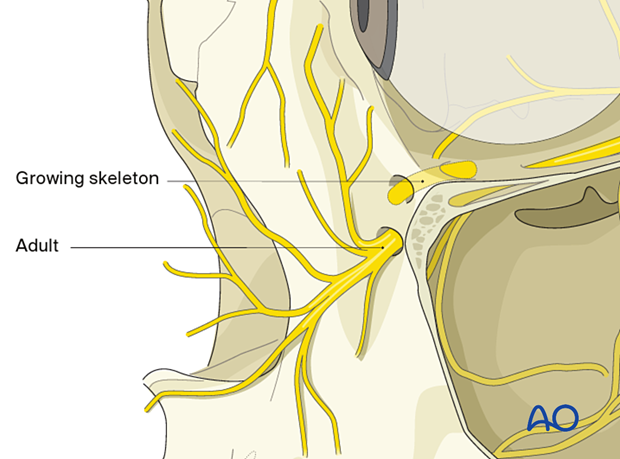
Forced duction test
After the insertion of an implant and before closure, it is imperative to perform a forced duction test and to examine the state of the pupil.
3. Choice of reconstruction material
General considerations
Unless prebent plates are used, the unique and complex anatomy of the orbit requires significant contouring of the implants to restore the proper anatomy.
Most cases require reconstruction of the orbital floor to support the globe position and restore the shape of the orbit. Therefore, the concept of orbital reconstruction is facilitated by replacing the missing bone with alloplasts rather than trying to reconstruct by reducing bone fragments. This can be accomplished using various alloplastic materials. The larger the defect, the more the use of contoured implants is indicated. Preformed anatomic implants, either patterned upon averages or patient specific, can facilitate precise restoration of orbital anatomy.
There is hardly any anatomic region in the human body as controversial as this one in terms of appropriate material for use in fracture repair:
- Nonresorbable versus resorbable
- Autogenous/allogeneic/xenogeneic versus alloplastic material
- Non-prebent versus preformed (anatomical) plates
- Standard versus patient-specific plates
- Nonporous versus porous material
- Noncoated versus coated plates
Many surgeons recommend using radiopaque materials that allow bending to a precise anatomical shape and intra- or postoperative radiologic confirmation of placement. These materials should also be stable over time.
The use of resorbable materials is not indicated. Use of resorbable materials may lead to secondary changes of the orbital contours over time, which is undesirable.
There is a paucity of evidence to support the ideal choice for an orbital implant.
Modern imaging analysis offers a unique chance to assess the surgical result and stability over time. This can provide valuable information for future recommendations.
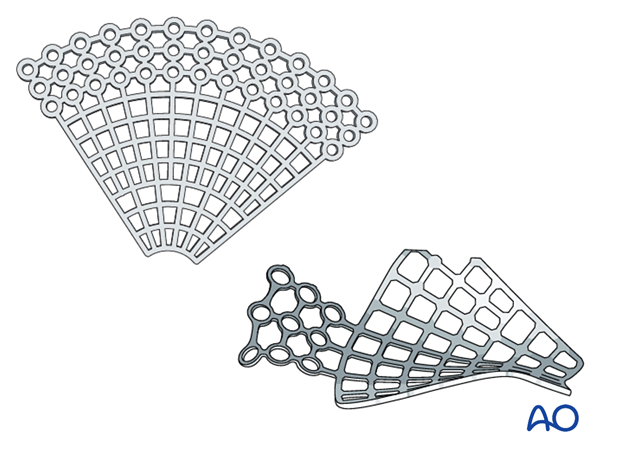
Titanium mesh
Advantages include the following:
- Availability
- Stability
- Contouring (aided using a sterile artificial skull)
- Ideal for large defects or three-wall fractures (the prebent plate is limited to medial wall and orbital wall fractures only)
- Radiopaque
- Spaces within the mesh to allow dissipation of fluids and blood
- No donor site needed
- Tissue incorporation may occur
Disadvantages include the following:
- Cost
- Possible sharp edges if not correctly trimmed
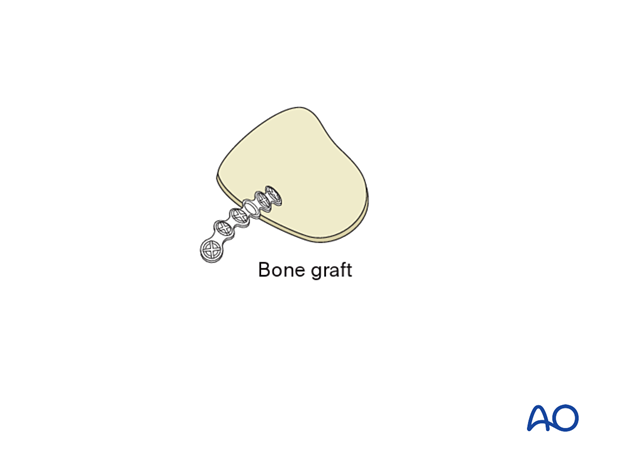
Bone graft
Advantages include the following:
- Low material costs
- Smooth surface
- Variability in thickness
- Radiopacity
- Maximal biocompatibility
- Periorbita readily dissects off from the bone in secondary reconstructions
The illustration shows a calvarial bone graft stabilized by a titanium fixation plate.
Disadvantages include the following:
- Additional donor site needed (necessitating extra surgery time for harvest, pain, scar, and possible surgical complications)
- Possible contour and dimensional changes due to remodeling
- Difficult to shape according to patient’s anatomy
- Less drainage from the orbit than with titanium mesh
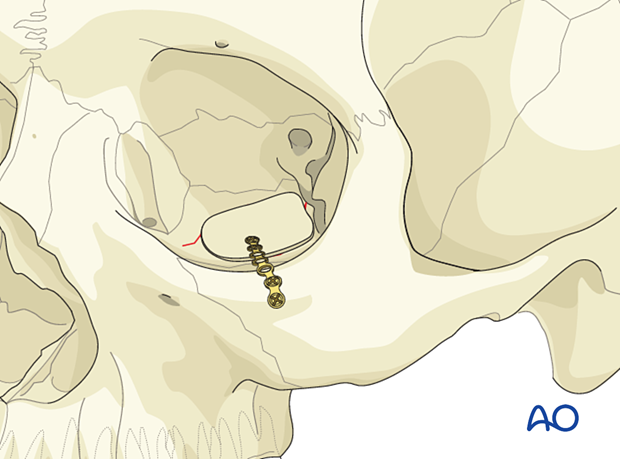
Porous polyethylene sheets (PPE)
Advantages include the following:
- Availability
- Contouring (eased by a sterile artificial skull)
- Smooth edges
- Allows tissue ingrowth
Disadvantages include the following:
- Not radiopaque (not visible on postoperative images)
- Lack of sufficient rigidity may produce improper support and contour when a very thin wafer of PPE is used
- Lack of proper contour in implant position creates disturbances in globe position
- Disturbances in globe position occur from implant malposition, improper contours, insufficient or excessive strength
- Less drainage from the orbit than with titanium mesh
- Difficult to reproduce normal orbital anatomy
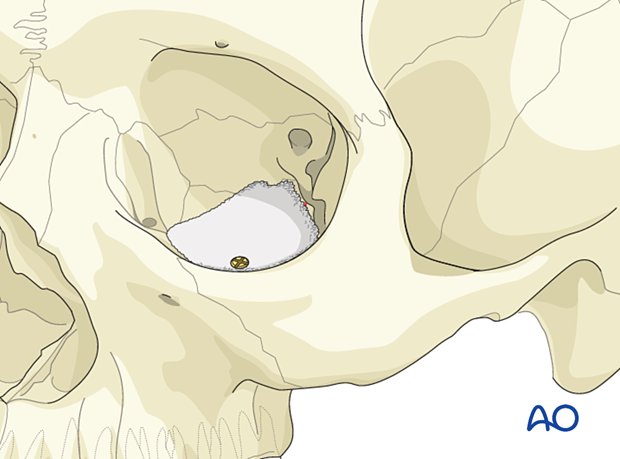
Composite of porous polyethylene and titanium mesh
AdvantagesA combination of titanium mesh and porous polyethylene is radiopaque and more rigid than porous polyethylene of a similar thickness. Some surgeons also believe that there is less risk of having retained sharp barbs, which can lead to entrapment of soft tissues during placement. This implant is designed to guarantee retention of a created shape, something which cannot be achieved by regular polyethylene without the memory provided by the titanium.
Other advantages are:
- Availability
- Stability
- Contouring (eased by a sterile artificial skull)
- Adequate in large three-wall fractures (the prebent plate is limited to medial wall and orbital wall fractures only)
- Radiopacity
- No donor site needed
- Tissue incorporation may occur
A composite of porous polyethylene and titanium mesh has the following disadvantage:
- Less drainage from the orbit than with titanium mesh
Resorbable materials (thermoplastic and non-thermoplastic)
Advantages include the following:
- Availability
- Handling/contourability (only for thermoplastics)
- Smooth surface and smooth edges
Disadvantages include the following:
- Non radiopaque
- Degradation of material with possible contour changes
- Sterile infection/inflammatory response
- Challenging to shape according to patient’s anatomy (only for non-thermoplastics)
- Non-perforated implants may not allow drainage of fluid or blood from the orbit
- Any material may have sharp edges if not properly contoured
The illustration shows a non-thermoplastic port delivery system (PDS) implant.
Preformed (anatomic) orbital implant
Advantages include the following:
- Radiopaque
- Smooth surface
- Minimal or no contouring is necessary
- Restores normal orbital anatomy
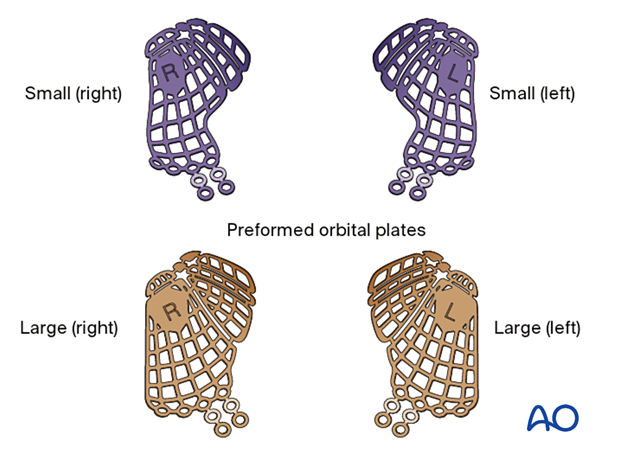
Disadvantages include the following:
- Cost
- May be difficult to properly position
- Each implant represents grouped orbital averages and may not be patient specific
This illustration shows a multiplanar and 3D view of a preformed mesh plate (STL) virtually placed before surgery into a patient CT dataset.
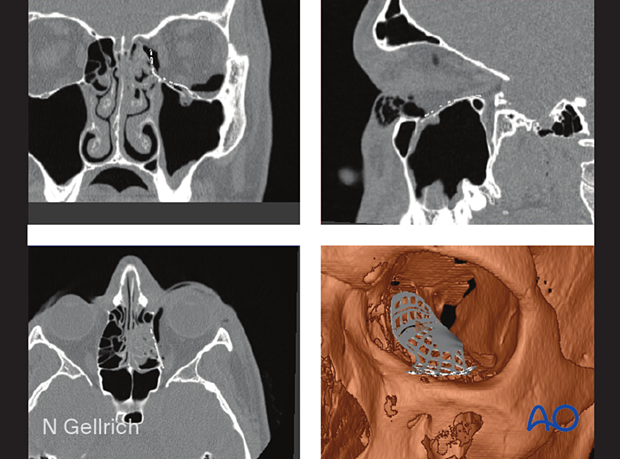
Patient-specific implant
A 3D printed patient-specific implant (PSI) can be manufactured using the patient’s own CT data to allow for the precise reconstruction of large orbital defects. Mirroring the uninjured orbit allows for the anatomic restoration of the fractured orbit during the planning process.
Advantages include the following:
- Radiopaque
- Smooth surface
- No contouring is necessary
- Restores normal orbital anatomy perfectly
Disadvantages include the following:
- Cost
- Time to manufacture
- Availability
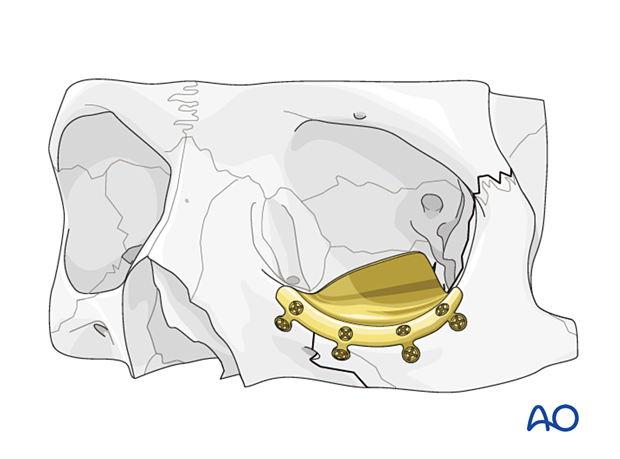
Implant fixation
Fixation of orbital reconstruction material varies according to the type and nature of the fracture.
Fixation of most materials in the orbital floor is achieved using one or more screws. The diameter depends on anatomical requirements but will typically vary between 1.0 and 1.5 mm. Alternatively, other screw types can be used.
4. Surgical exposure
Periorbital dissection
The orbital floor can be reached via various lower eyelid approaches (transcutaneous or transconjunctival).
Once the orbital floor is exposed, periorbital dissection is performed.
Additional information about orbital anatomy and dissection can be found in the links below:
- Preoperative considerations
- Anatomy of the bony orbit
- Correlation of surface and cross-sectional anatomy
- Introduction to periorbital dissection
- Orbital floor dissection
- Medial orbital wall dissection
- Lateral orbital wall dissection
- Orbital roof dissection
- Adjunctive access procedures (orbitotomies)
- Retrobulbar hemorrhage
Retraction
Adequate exposure and illumination (headlights, illuminated retractors) of the fractured area is imperative before fixation.
Adequate hemostasis must be achieved.
Appropriate retraction of the intraorbital soft tissues must be performed.
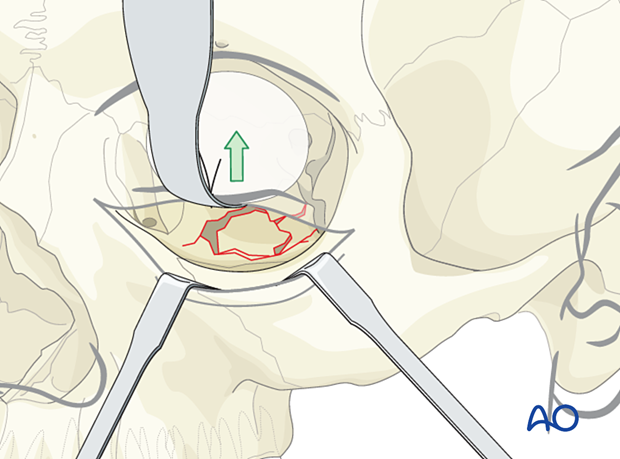
A foil (or sheet of other material) with a retractor may help avoid a prolapse of soft tissues, improve visualization, and prevent entrapment of soft tissues during implant placement.
The illustration shows the insertion of a foil below the retractor.
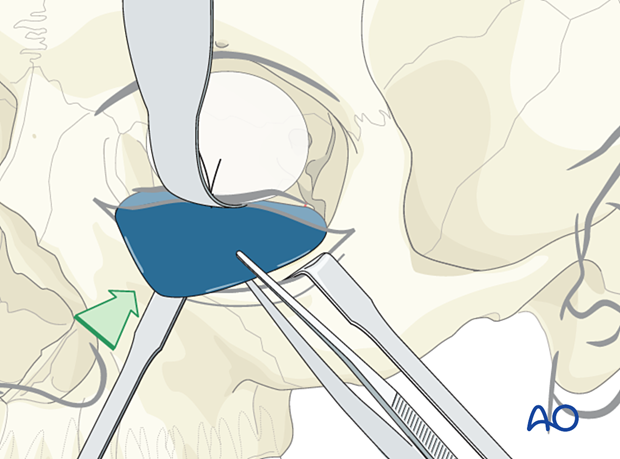
The retractor is removed, placed under the foil, and the orbital soft tissues are adequately retracted.
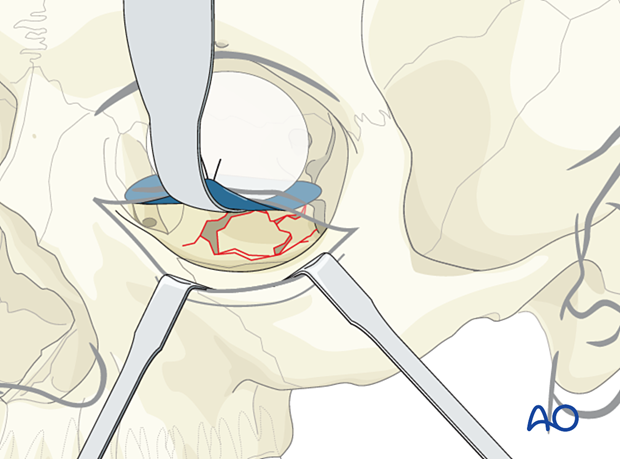
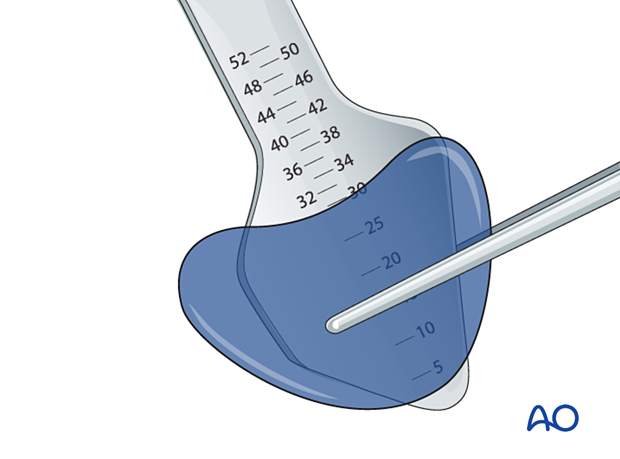
Helpful devices
Many devices have been used to facilitate retraction of the orbital contents, including malleable retractors, spoons, and special orbital retractors designed for the globe (as illustrated).
5. Reconstruction
Measurement of defect size and determination of implant size
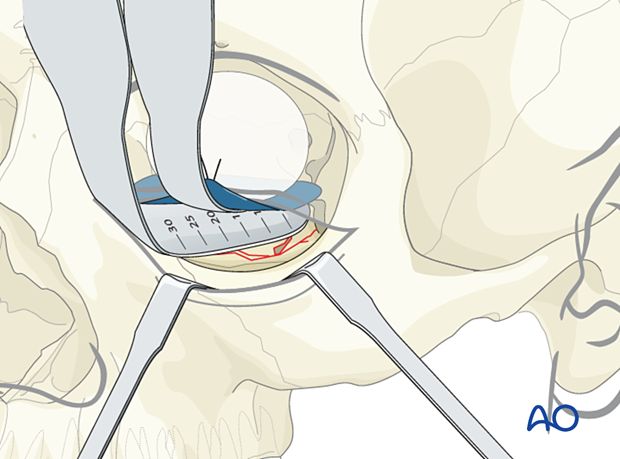
A careful assessment of the defect size should be performed preoperatively with a CT scan from several views. The sagittal view, which is in the longitudinal projection of the optic nerve, plus the coronal view showing the transverse extent are especially helpful.
It is crucial that in the case of combined fracture types (eg, displaced zygoma fracture), the final defect size is only measured after the reduction of the zygoma. The defect size can be measured with the reading on the orbital retractor or another measuring instrument (ie, ruler).
In general, the mesh should not extend anteriorly over the orbital rim. Any metallic or alloplastic fixation material may create problems due to undesirable fit, size, and thickness with the position and function of the lower lid.
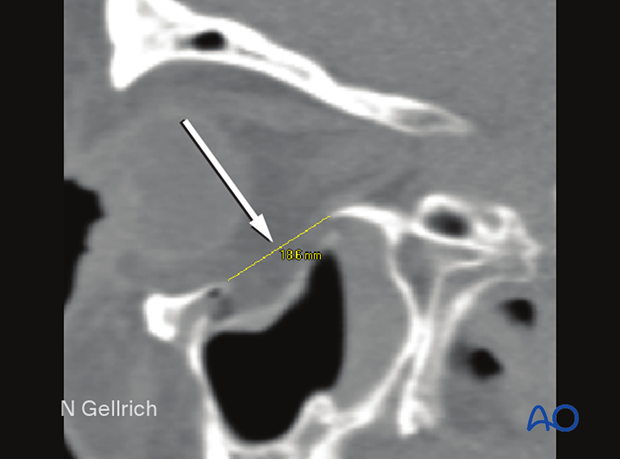
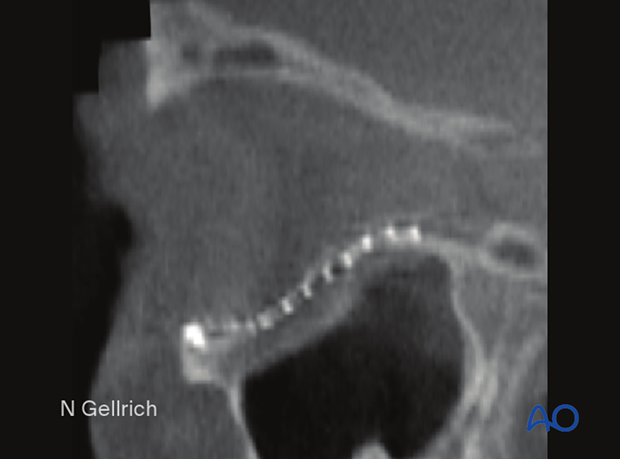
Preoperative CT showing the extent of an orbital floor fracture in sagittal view.
Postoperative CT showing the S-shaped recontouring of the orbital floor.
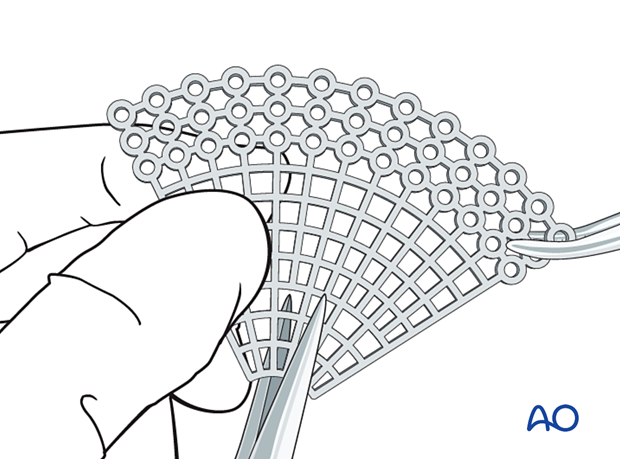
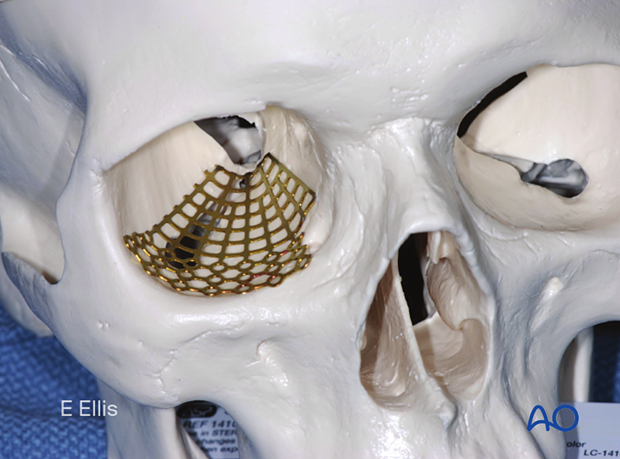
Example of reconstruction with titanium mesh
Firstly, the mesh is cut.
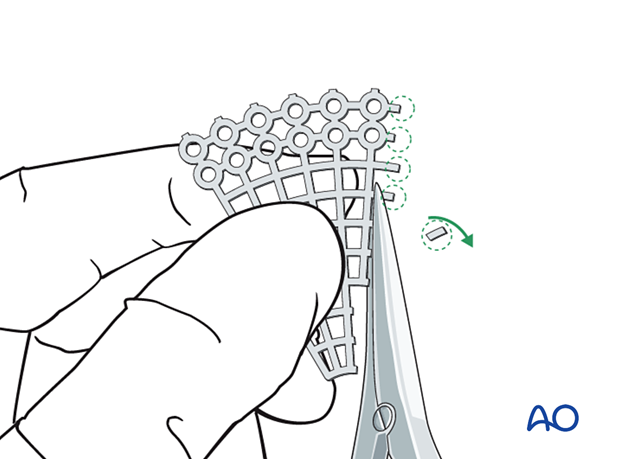
Then all sharp edges of the plate are trimmed off to protect the soft tissues (note the shape of the fan has only a minimum number of screw holes).
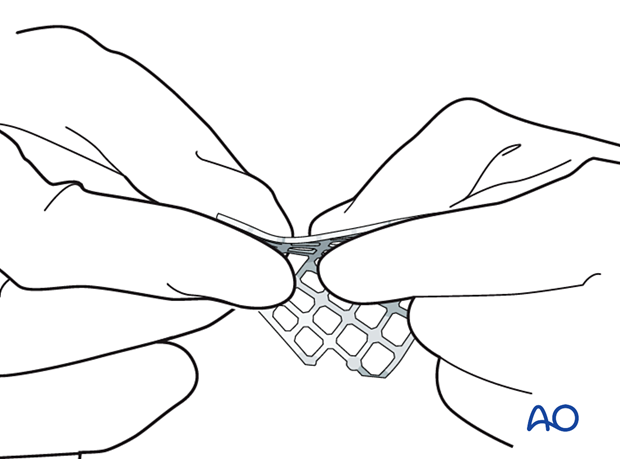
After that, the mesh is contoured to achieve the required shape and accommodate key anatomical structures (nasolacrimal duct, infraorbital nerve, and optic nerve).
It is advisable not to extend the implant further posterior than 5mm anterior to the optic canal entrance (if the posterior support bone of the orbit can be reached).
A sterile artificial skull may be used for anatomical contouring of the implant. As shown in this image, the entire implant can be contoured to the shape required for an inferior orbit. This contoured implant can then be used to cover a range of inferior orbital defects. Individual modification of an implant for size, shape and position is always required to fit the needs of the individual patient. The sequence of inserting, measuring, withdrawing, and further modifying the implant can be repeated until an implant of the proper size and shape is produced.
Post-insertion modification may be minimized if a correctly-sized pre-shaped mesh is available.
- When using a fan-shaped plate, be aware that the mesh is designed to cover almost any reconstruction need. The width of the mesh should be modified to fit the defect. It should be as small as possible with the edges of the mesh overlapping the edges of the defect.
- The need for screw fixation varies with the material used and the shape and contours of the anatomic area. As a general principle screw fixation is preferred.
- It is essential to create a plate that is large enough to span the defect and to permit recreation of the contour of the orbit in that location.
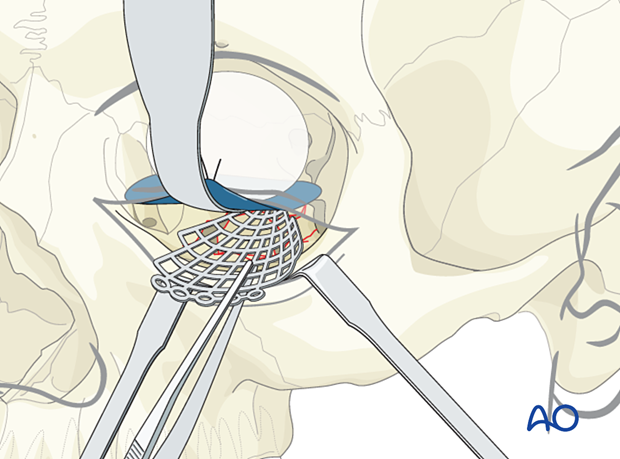
Regardless of the implant chosen, the insertion process must prevent deformation of the contour of the mesh. While the intraorbital soft tissues are adequately retracted, the mesh must be positioned so that proper and stable recontouring of the orbital walls takes place. During the insertion process, care should be taken that neither orbital fat nor muscles are entrapped. After the initial insertion, a specific step should be achieved which dissects the entire anterior surface of the implant and frees any entangled muscle or soft tissue. The mesh may require rotation to be correctly positioned in a passive position. A forced duction test must be performed after the insertion to demonstrate the range of free globe motion. Any impairment requires immediate correction.
Option: Computer assisted surgery
DICOM data can be used to generate 3D virtual models of the patient’s orbit, and a CAD-CAM custom shaped implant can be fabricated.
Further information about Computer Assisted Surgery can be found here.
Navigation
Additionally, navigation may serve for intraoperative control of implant or fragment position.
Modern 3D C-arm technology will further improve intraoperative quality control of implant positioning. Click here for more information.
Endoscopic visualization
Endoscopic visualization, either through the maxillary sinus or intraorbitally, may additionally confirm implant placement and lack of incarceration of periorbital soft tissue.
6. Fixation
Mesh fixation
If fixation is required, one screw will be enough in most cases. The screw can be placed in solid bone such as the anterior floor of the orbit just posterior to the orbital rim. The screw and the mesh must be compatible with size and metal type.
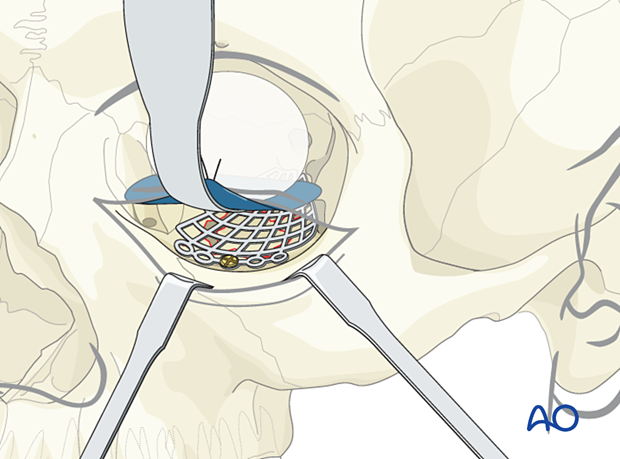
Alternatively, the mesh can be positioned, and a screw can be placed on the anterior face of the maxilla.
Internal orbital screw placement is preferred to avoid excess soft-tissue reaction to metals, palpability, and visual silhouette at the rim.
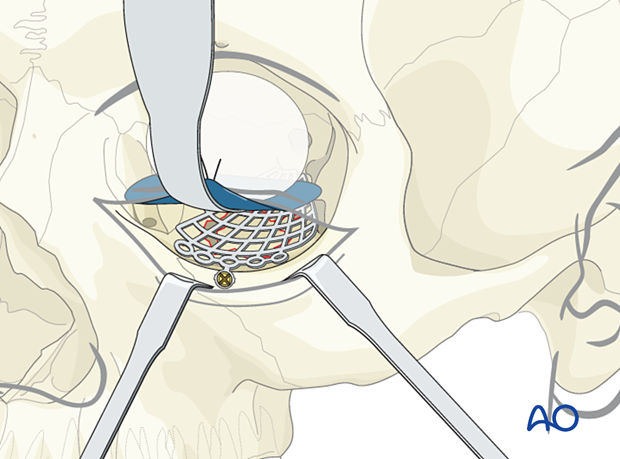
Forced duction test
After the insertion of an implant and before closure, it is imperative to perform a forced duction test and to examine the state of the pupil.

Alternative: Bone graft
If bone is harvested from a donor site, eg, cranial vault (parietal area), iliac crest, mandible, rib, etc, there may be additional donor site morbidity.
According to the defect dimensions, the bone graft must be adapted and fixed either with screw fixation only or with plate and screw fixation (as illustrated).

7. Case example
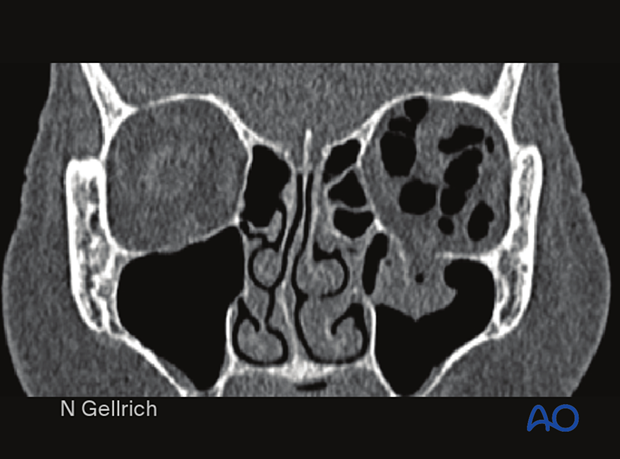
Coronal CT (bone window) of an isolated left orbital floor fracture. Note the extensive intra-orbital emphysema.
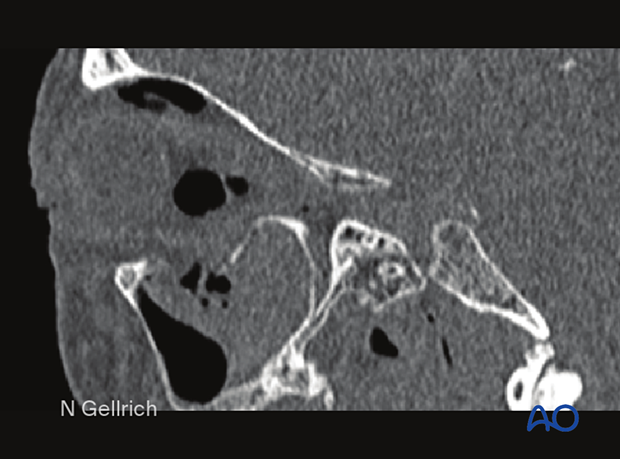
Sagittal CT slices (bone window) showing a large defect in the orbital floor with herniation of the periorbita into the maxillary sinus.
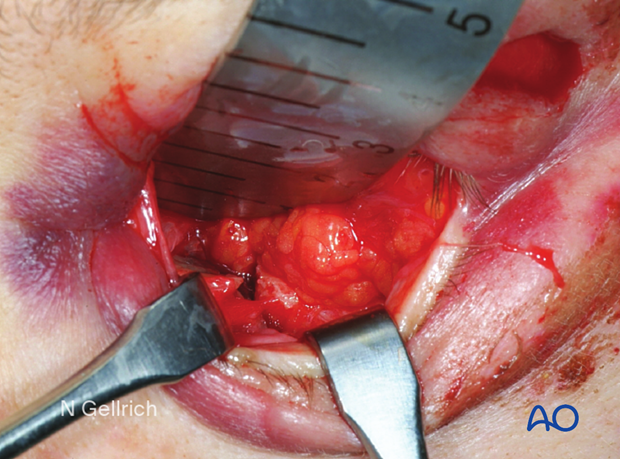
Transconjunctival incision and exposure of the fracture.
Attempted repositioning of the fracture fragment.
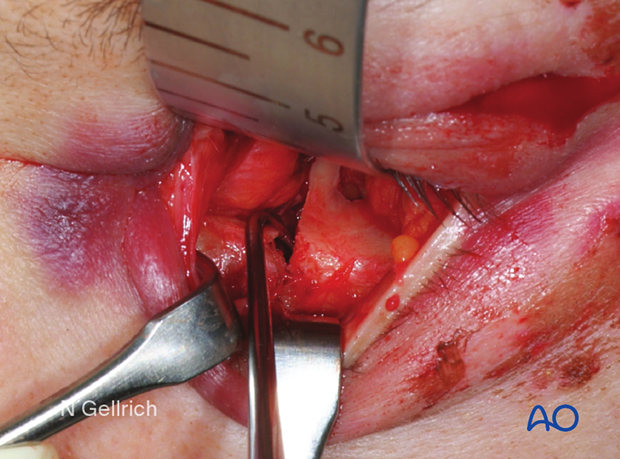
Removal of the displaced orbital floor fragment.
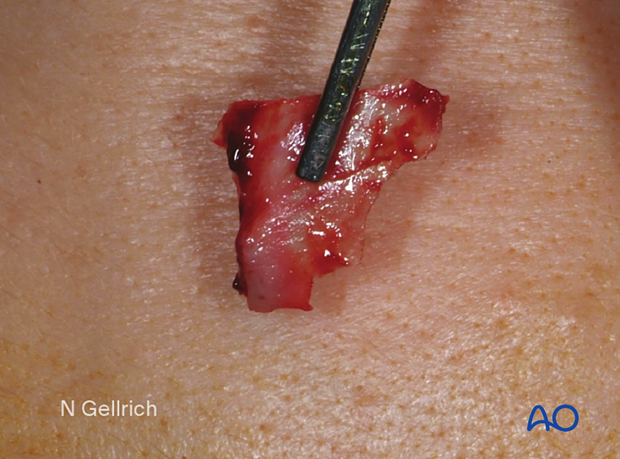
A sterile artificial skull is used intraoperatively to aid in contouring the plate to fit the shape of an orbit.
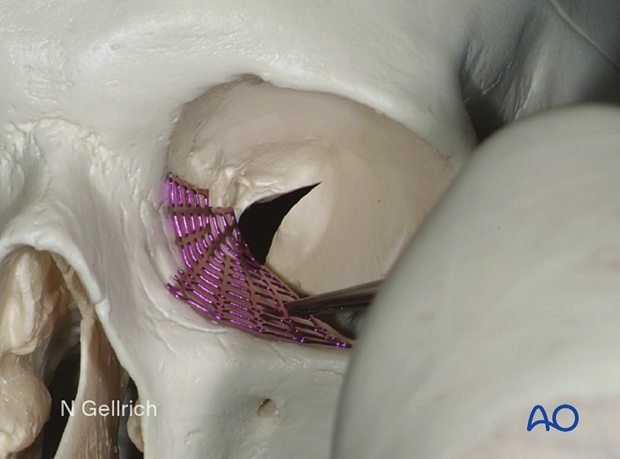
The mesh is bent.

The bulges are formed.

The proper contour is checked.
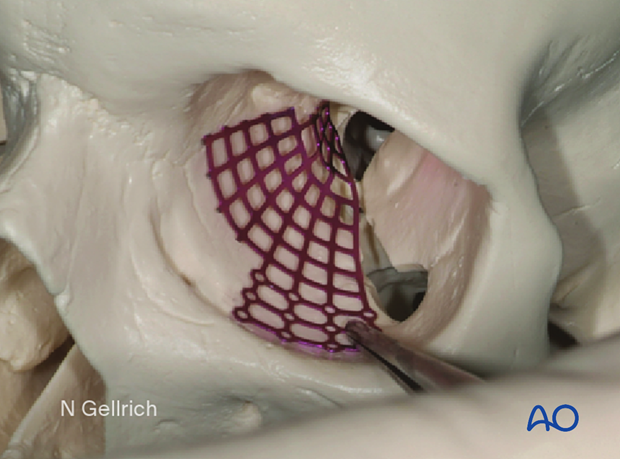
Exposure before mesh insertion.
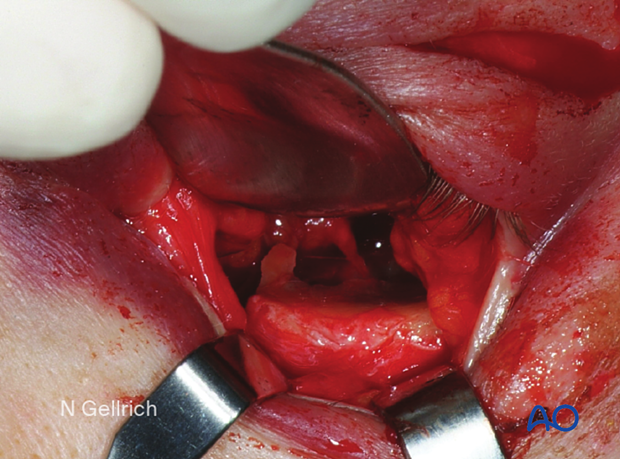
The implant is inserted and marked to facilitate trimming. It is then then withdrawn and trimmed to fit the defect.
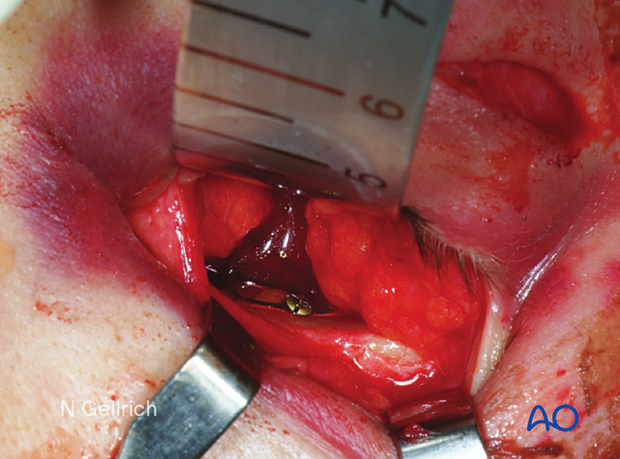
The trimmed mesh is then inserted and held in place.
It is important to visualize the entire mesh at this point to ensure that the implant is properly positioned, and there is no entrapment of the periorbita.
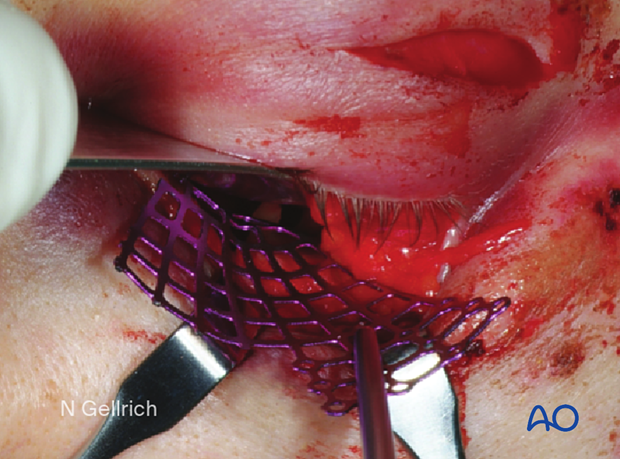
Single screw fixation posterior to the orbital rim.
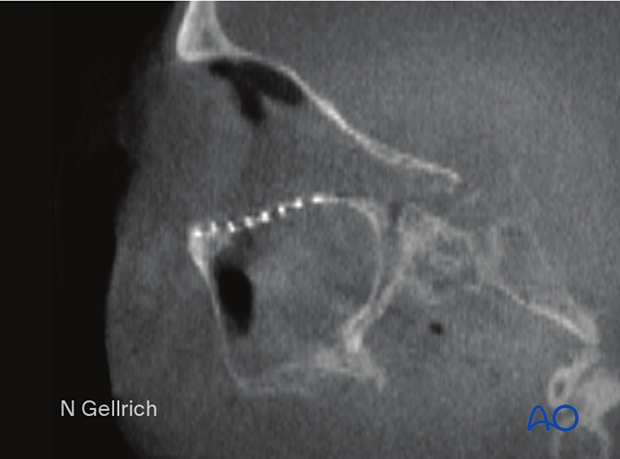
Postoperative sagittal cone-beam CT slice of an isolated left orbital floor fracture treated with a fan-shaped titanium mesh. Note the anatomic shape of the implant covering the entire defect and resting on the posterior ledge.
8. Aftercare
Evaluation of the patient’s vision
Patient vision is evaluated on awakening from anesthesia and then at regular intervals until hospital discharge.
A swinging flashlight test may serve to confirm pupillary response to light in the unconscious or non-cooperative patient; alternatively, an electrophysiological examination must be performed but this is dependent on the appropriate equipment (VEP).
Postoperative positioning
Keeping the patient’s head in a raised position both preoperatively and postoperatively may significantly reduce edema and pain.
Nose blowing
Nose blowing should be avoided for at least ten days following fracture repair to prevent orbital emphysema.
Medication
The use of the following perioperative medication is controversial. There is little evidence to make solid recommendations for postoperative care.
- No aspirin for seven days (nonsteroidal anti-inflammatory drugs (NSAIDs) use is controversial)
- Analgesia as necessary
- Antibiotics: many surgeons use perioperative antibiotics. There is no clear advantage of any antibiotic, and the recommended duration of treatment is debatable.
- A nasal decongestant may be helpful for symptomatic improvement in some patients.
- Steroids, in cases of severe orbital trauma, may help with postoperative edema. Some surgeons have noted increased complications with perioperative steroids.
- Ophthalmic ointment should follow local and approved protocol. This is not generally required in the case of periorbital edema. Some surgeons prefer it. Some ointments have been found to cause significant conjunctival irritation.
Ophthalmological examination
Postoperative examination by an ophthalmologist may be requested. The following signs and symptoms are usually evaluated:
- Vision
- Extraocular motion (motility)
- Diplopia
- Globe position
- Visual field test
- Lid position
- If the patient complains of epiphora (tear overflow), the lacrimal duct must be checked
- If the patient complains of eye pain, evaluate for corneal abrasion
Postoperative imaging
Postoperative imaging must be performed within the first days after surgery. 3D imaging (CT, cone beam) is recommended to assess complex fracture reductions. An exception may be made for centers capable of intraoperative imaging.
Wound care
Ice packs are effective in the short term to minimize edema.
Remove the sutures from the skin after approximately five days if non-resorbable sutures have been used.
Avoid sun exposure and tanning to skin incisions for several months.
Diet
Diet depends on the fracture pattern.
A soft diet can be taken as tolerated until adequate healing of the maxillary vestibular incision.
Clinical follow-up
Clinical follow-up depends on the complexity of the surgery and whether the patient has any postoperative problems.
With patients that have fracture patterns that include periorbital trauma, issues to consider are the following:
- Globe position
- Double vision
- Other vision problems
Other issues to consider are:
- Facial deformity (including asymmetry)
- Sensory nerve compromise
- Problems of scar formation
Eye movement exercises
Following orbital fractures, eye movement exercises should be considered.
Implant removal
Generally, implant removal is not necessary except in the event of infection or exposure.
Follow-up
The patient needs to be examined and reassessed regularly. Follow-up imaging at 3–6 months is helpful to ensure proper pneumatization of the sinuses (particularly, mucocele formation must be ruled out), sealing of the skull base, and stability of fragment position.
Special considerations for orbital fractures
Travel in commercial airlines is permitted following orbital fractures. Commercial airlines pressurize their cabins.
Facial fractures may predispose to Eustachian tube dysfunction due to pharyngeal swelling. Forced air insufflation by holding the nose, closing the mouth and attempting expiration and the use of decongestants can relieve middle ear pressure and drum discomfort.
Mild pain on ascent or descent in airline travel may be noticed. Flying in unpressurized aircraft, such as military planes, should be avoided for a minimum of six weeks.
No scuba diving should be permitted for at least six weeks.













