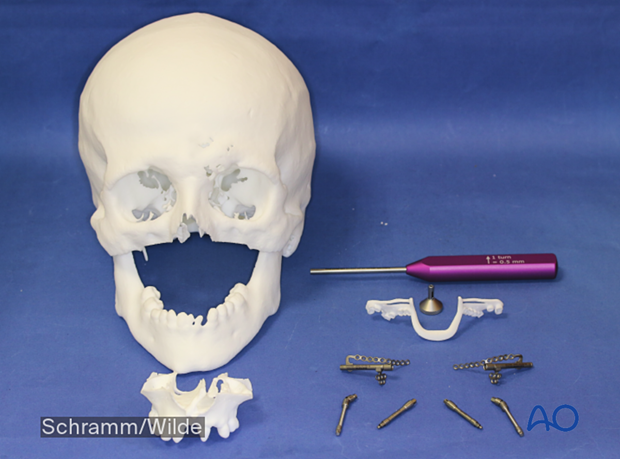
An introduction to computer assisted surgery
1. What is computer assisted surgery?
Computer assisted surgery uses computers to manipulate Digital Imaging and Communication in Medicine (DICOM) data for planning, performing, and assessing surgical procedures.
Computer assisted surgery can be broken down into three basic steps: presurgical planning, intraoperative assistance, and postoperative applications.
The benefits of presurgical planning include:
- Improved diagnostic accuracy
- Preoperative surgical planning
- Virtual simulation of the surgical procedure
Intraoperatively, the main benefit of computer assisted surgery is improved accuracy of the surgical repair. This results from the use of:
- Intraoperative CT
- Patient-specific implants such as preformed implants based on patient-specific 3D models, and CAD/CAM implants fabricated from PEEK, ceramic, or titanium (milled or laser-sintered)
- Intraoperative surgical guides (drill guides, cutting guides, patient-specific osteosynthesis)
- Intraoperative navigation
Postoperatively, an assessment of the accuracy of a surgical procedure is facilitated.
The disadvantages of computer-aided surgery are primarily related to the cost of the hardware and software. Significantly more time is needed for training and using the planning software than is required by traditional techniques.
Some techniques currently in routine clinical use have led to reduced OR time, eg, maxillary positioning in orthognathic surgery.
2. Planning software
There are currently multiple software packages available for presurgical planning, with more to come in the future. These include both open source and commercially-available software.
Furthermore, planning software should allow for the import and export of stereolithography (STL) or similar 3D files.
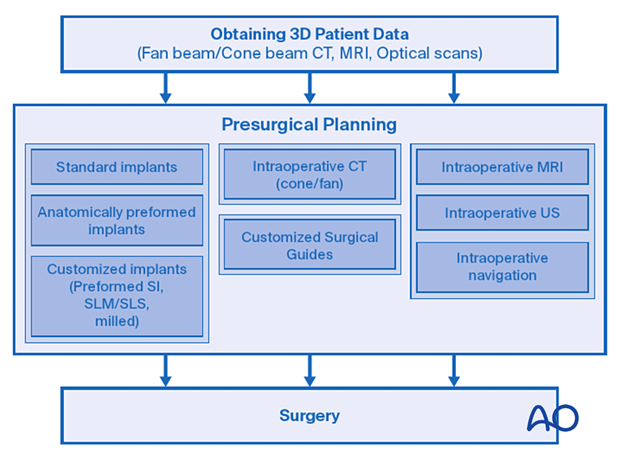
3. General workflow
This illustration incorporates pre- and intraoperative applications into a common surgical workflow. Patient 3D data is obtained, presurgical planning is performed, and the intraoperative application is selected based on the procedure's complexity. Complex cases may start with 3D patient-specific virtual models, surgical guides and templates, and patient-specific implants, followed by intraoperative imaging and navigation.
4. Virtual planning
Data acquisition
Appropriate imaging data is obtained before surgery. This data is then imported into the planning software.
This may include a fan beam or cone beam CT scan, MRI, surface laser scan, intraoral optical scan, etc.
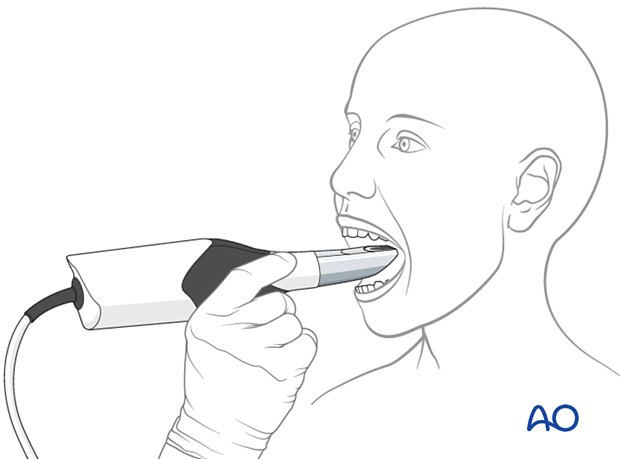
This illustration shows a 3D intraoral optical scan.
Segmentation
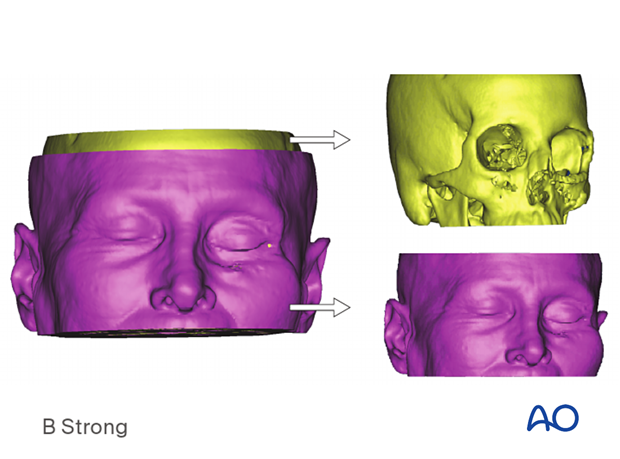
The illustration shows segmentation using Hounsfield units (radiodensity) to divide a data set into bone and soft-tissue virtual models.
Further segmentation can be done manually or semi-automatically, allowing the surgeon to manipulate a defined anatomic structure virtually.
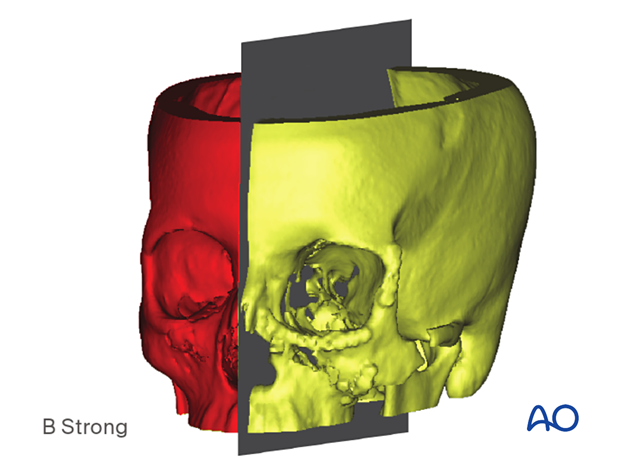
Segmentation and mirroring can be rapidly performed by dividing an anatomic structure (object) along a cutting plane.
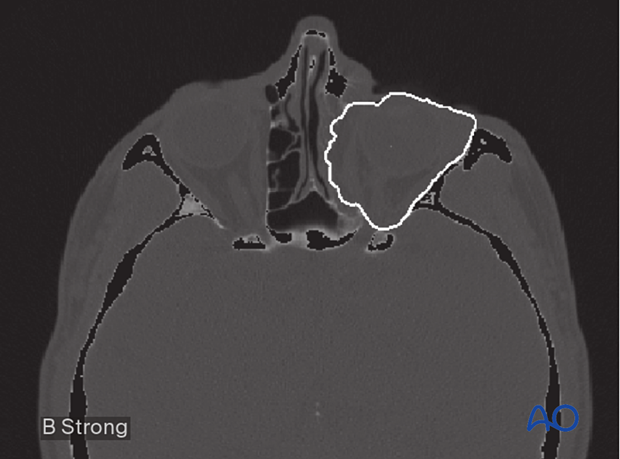
Complex segmentations, such as the orbit or mandible, involve producing an outline of the anatomic structure by stacking sequential axial DICOM slices to create a 3D volume. This is very labor-intensive and not conducive to routine clinical workflow.
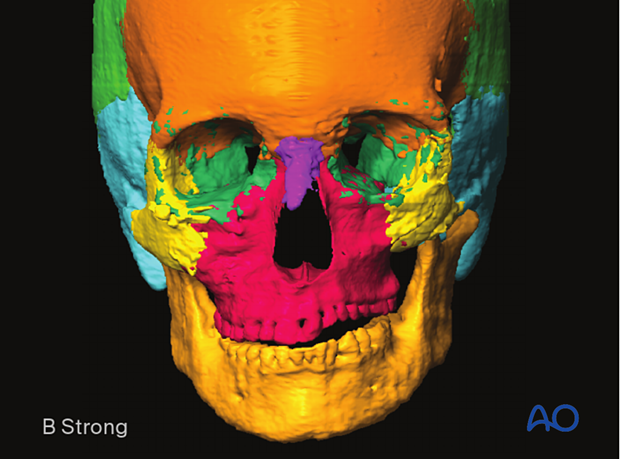
Semiautomatic segmentation using machine learning and artificial intelligence has been developed for the segmentation of more complex structures. It allows the computer to automatically segment an anatomic structure into predefined objects such as a maxilla, orbit, frontal bone, etc.
The image shows auto-segmentation into:
- Orbit (left/right)
- Maxilla
- Zygoma (left/right)
- Mandible
- Frontal bone
- Temporal bone (left/right)
Preoperative analysis
There is a broad range of measurements that can be performed during the preoperative analysis.
These include distance measurements.
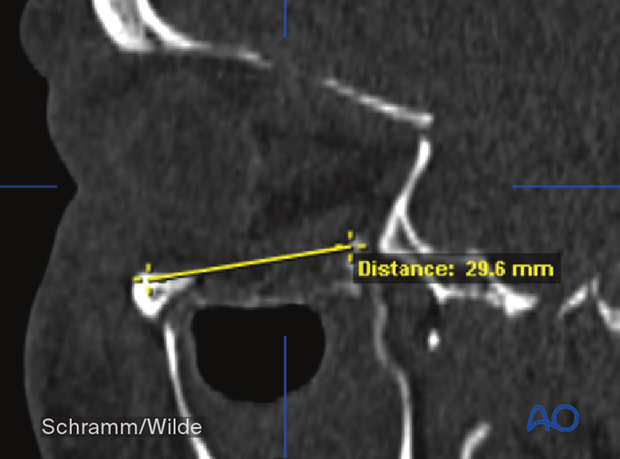
Angle measurements can also be performed during the preoperative analysis.
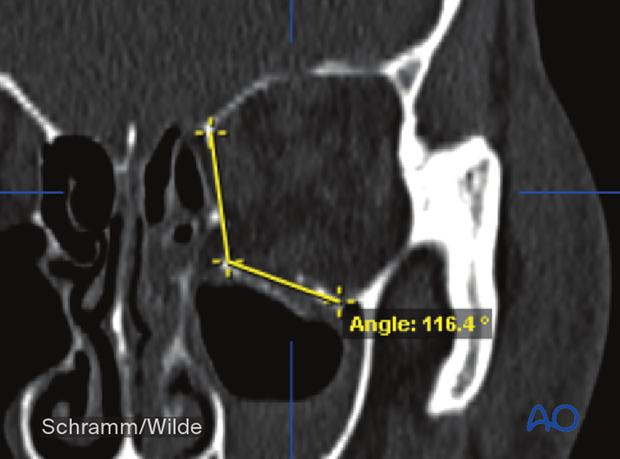
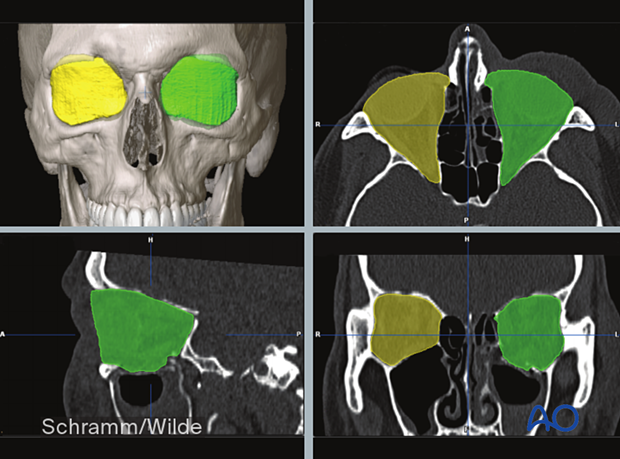
Volumetric measurements can also be performed during the preoperative analysis.
Presurgical planning software moves control of data presentation from the hands of the radiologist to the surgeon. He/she can rotate and visualize the virtual model with three degrees of freedom, offering an accurate depiction of depth, width, and projection. The surgeon can assess bony defects or deformities that would otherwise be very difficult to perceive in a 2D image.
Editing
Editing functions allow the surgeon to optimize the virtual model by erasing, adding, or moving structures.
Some examples might include:
- Removal of unnecessary peripheral structures to isolate an anatomic region
- Adding thickness to the bony orbit to ensure that a stereolithographic model is strong enough to allow the shaping of hardware
- Refining the contour of the bony orbit
Mirroring
This function allows the surgeon to mirror the patient's normal anatomy, from the non-injured side to the injured side on the virtual model. Hence, it is mainly useful for unilateral injuries/deformities.
It is crucial that the surgeon defines a symmetric mirror plane so that the uninjured template can be mirrored accurately over the injured anatomy. Some manipulation and refinement will be necessary.
It is also important for the surgeon to make a large enough “template” that extends beyond the area of injury to help align the mirrored object with the uninjured anatomy on the affected side.
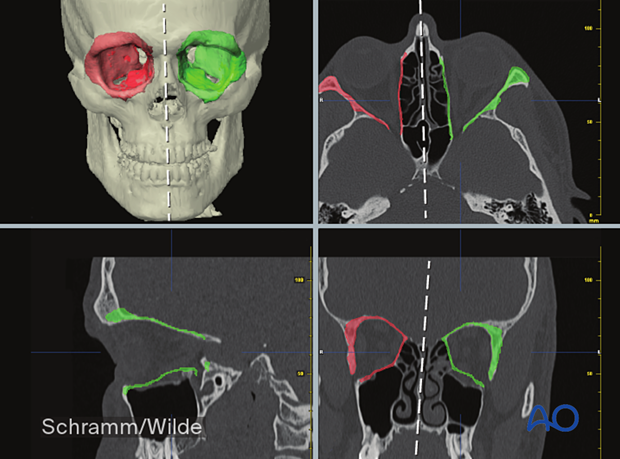
Presurgical planning/Virtual surgery
The surgical procedure is then planned in a virtual environment.
Planning may involve the determination of tumor resection margins, as shown here.
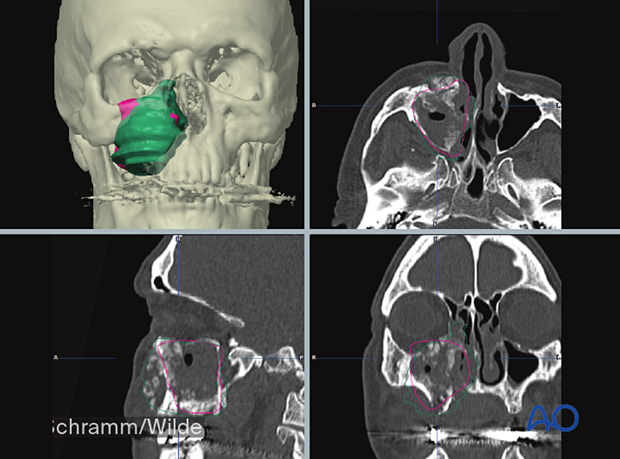
Osteotomies and occlusal corrections can also be planned.
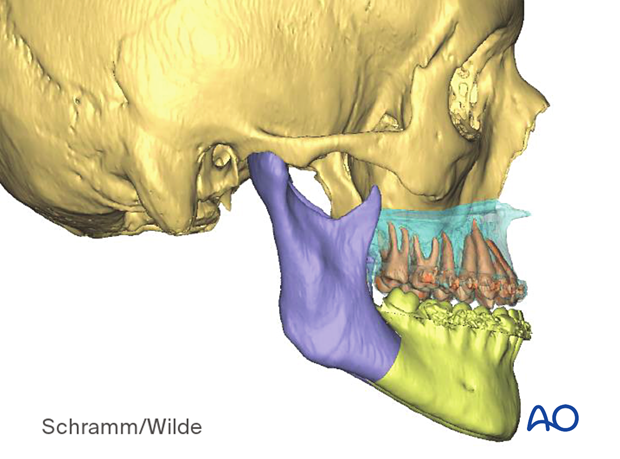
Presurgical planning can also be used for fracture reduction.
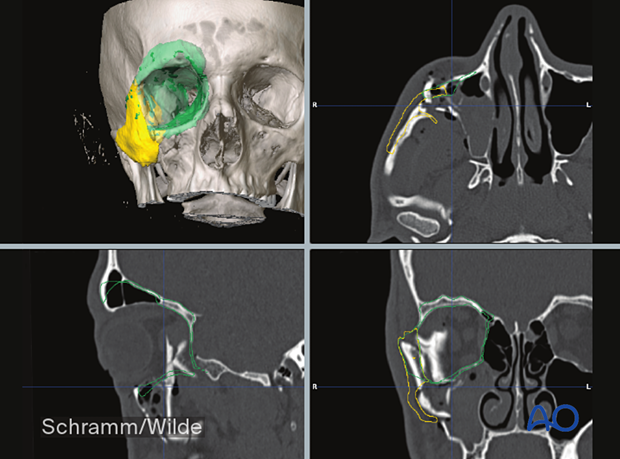
Image fusion
Image fusion is used to precisely overlay two different datasets. It allows the surgeon to analyze anatomic differences between the datasets accurately. Image fusion can be performed with two different CT data sets. It is most commonly used for pre-, intra-, and postoperative analysis (quality control).
A fusion of MRI and CT data sets is commonly performed for tumor surgery.
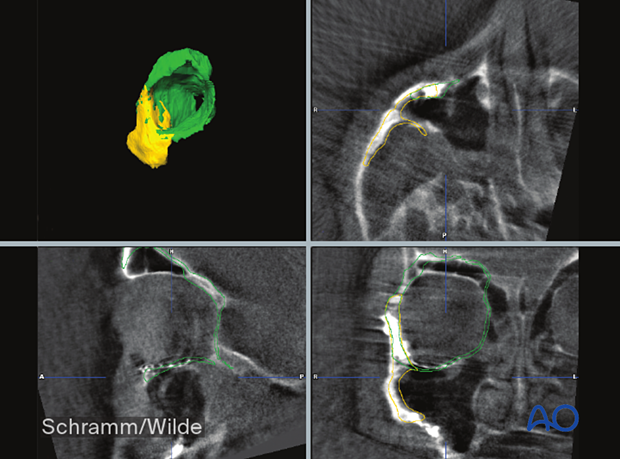
5. Intraoperative applications
Intraoperative CT
Intraoperative CT gives definitive anatomic information about bony reduction or metallic implant placement. It is generally performed when the surgeon is confident of the repair, as repeated CT scans may result in excessive x-ray exposure.
The fusion of virtual planning and intraoperative CT scans allows for precise evaluation of bony reduction, bony reconstruction, and implant placement.
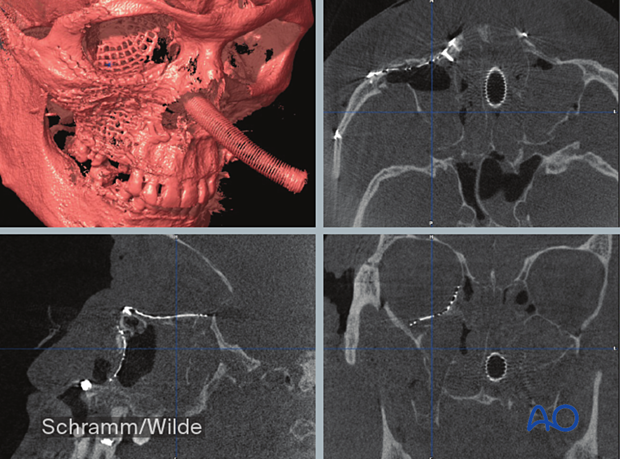
Intraoperative navigation
Intraoperative navigation is an interactive process that facilitates anatomically correct dissections and radical tumor resections by displaying invisible tumor borders.
Intraoperative navigation also allows confirmation of bony reduction and implant positioning. A trackable probe is moved along the bone/implant contour while the position is confirmed on the monitor. Navigation provides anatomic information without the use of x-rays. While the information is not as robust as intraoperative CT, navigation can be used as often as needed until the implant is found to be in the planned/accurate position without exposing the patient to radiation.
Anatomic 3D models
Rapid prototype models can be fabricated commercially or in-house to provide a physical 3D-representation of the patient preoperatively or after virtual planning of the surgery. They are typically used for complex reconstructions in which presurgical hardware shaping is beneficial. The modification of these models can be done in the following ways:
- Virtual simulation before 3D printing
- Surgical procedures performed on a printed patient-specific anatomic 3D model
- Preoperative fabrication of patient-specific implants and surgical guides can then be performed using these 3D models preoperatively
- Intraoperative fabrication of patient-specific implants and surgical guides can be performed if the fabrication processes allow for their sterilization and intraoperative use
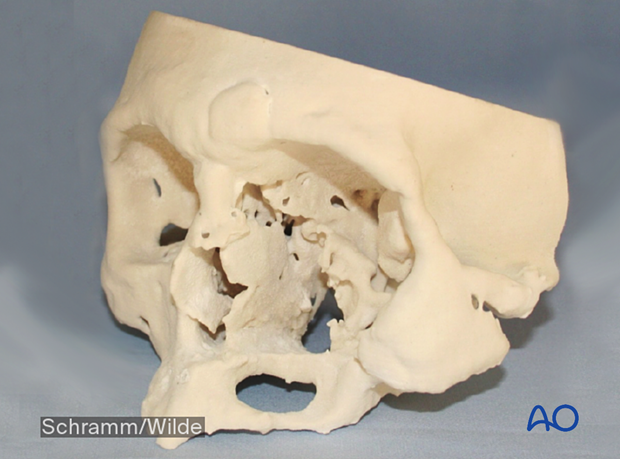
This image shows virtual simulation before 3D printing.
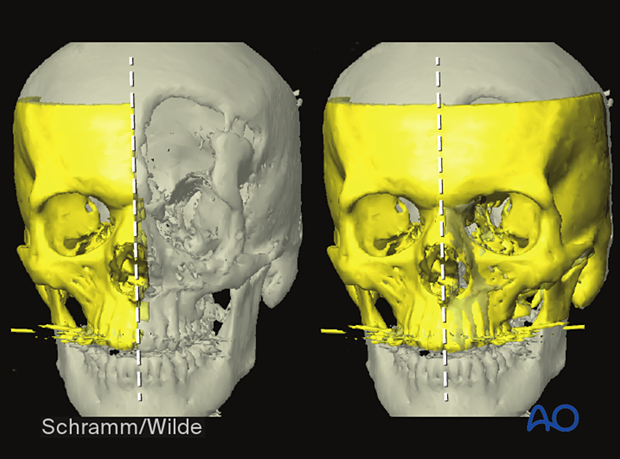
Surgical procedures can be performed on a printed patient-specific anatomic 3D model, shown here.
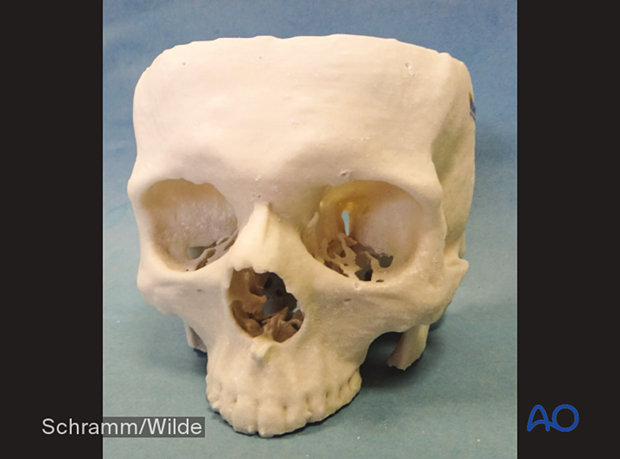
Preoperative fabrication of patient-specific implants and surgical guides can be carried out.
Intraoperative fabrication of patient-specific implants and surgical guides can also be performed.
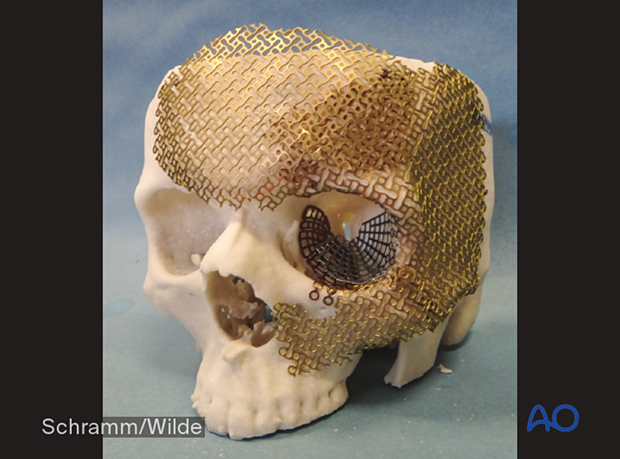
Patient-specific surgical guides and templates
Guides and templates are generally used intraoperatively to assist accurate osteotomies and precise placement of the bony segments. This technique can be used for a number of techniques, including the following:
- Maxillary and mandibular positioning in orthognathic surgery
- Mentoplasty in orthognathic surgery
- Mandibular reconstruction with a fibula flap
- Mandibular reconstruction with a DCIA flap
- Mandibular reconstruction with a scapula flap
- Midface reconstruction in trauma
- Ablative surgery
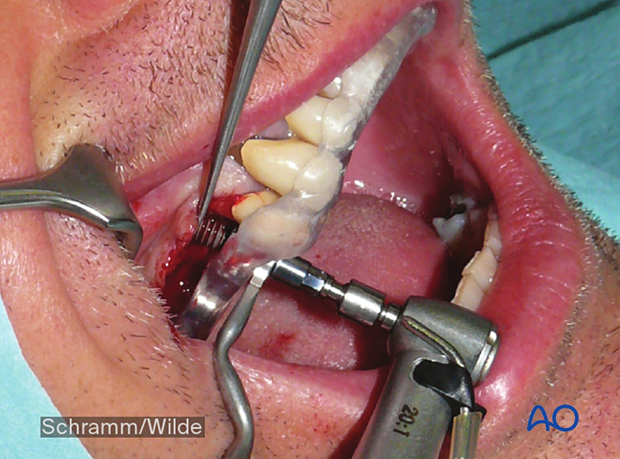
- Oral implant placement
- Correcting craniofacial malformation
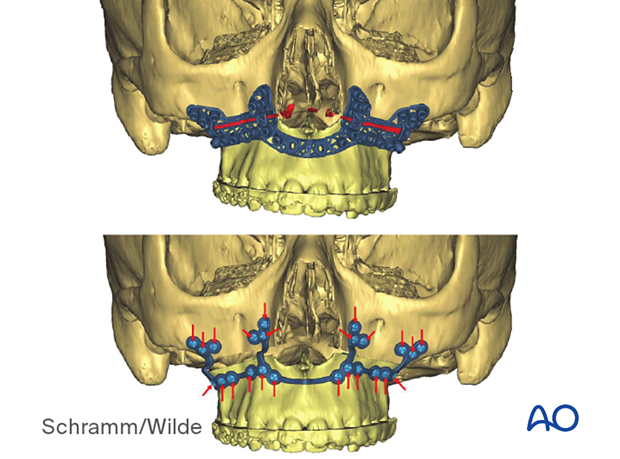
Patient-specific surgical guides and templates can be used for mentoplasty in orthognathic surgery.
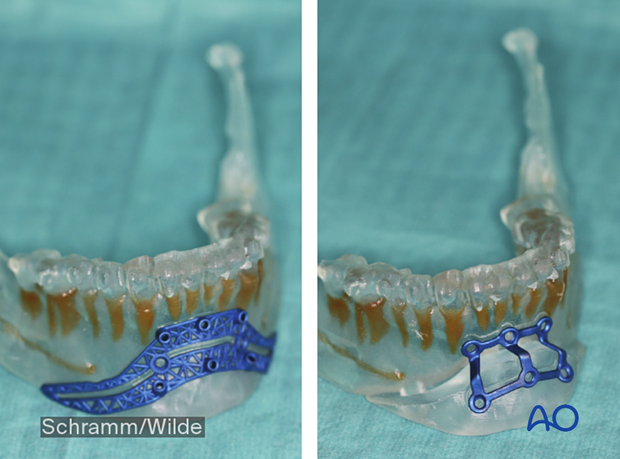
Patient-specific surgical guides and templates can be used for mandibular reconstruction with a fibula flap.
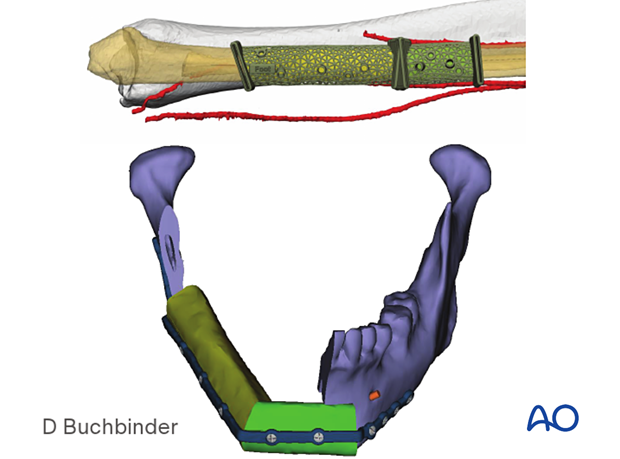
Patient-specific plates can be used to secure a DCIA flap.
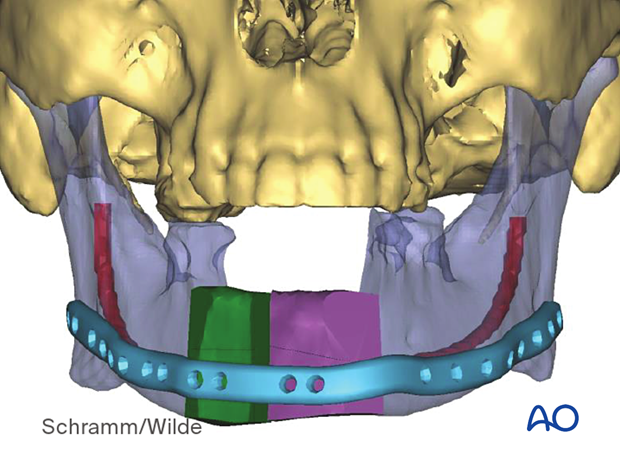
Patient-specific surgical guides and templates can be used for midface reconstruction in trauma.
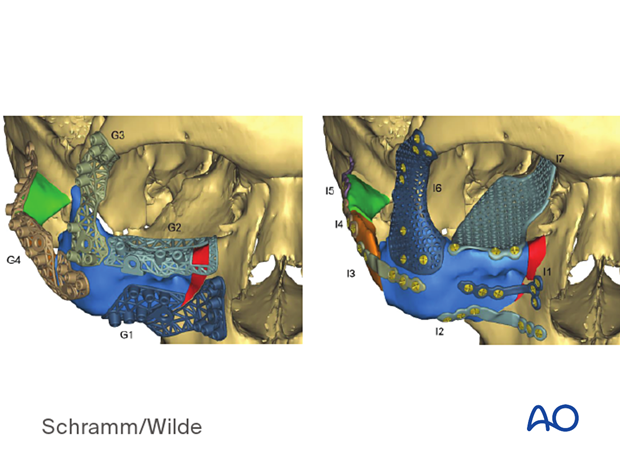
Patient-specific surgical guides and templates can be used for ablative surgery.
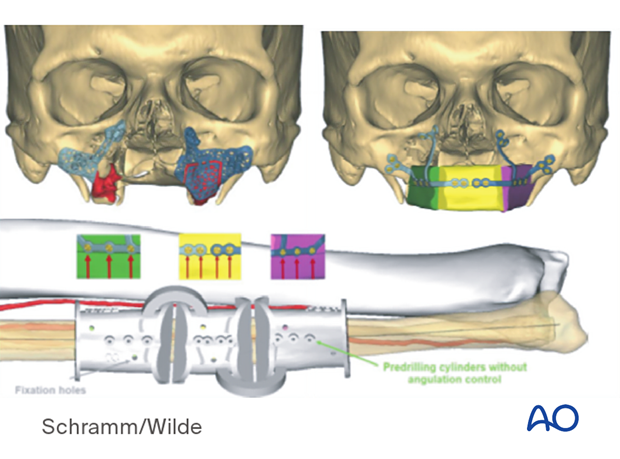
Patient-specific surgical guides and templates can be used for dental implant placement.
Patient-specific implants
Patient-specific implants are fabricated to either fill a bony defect or accurately reconstruct a bony contour.
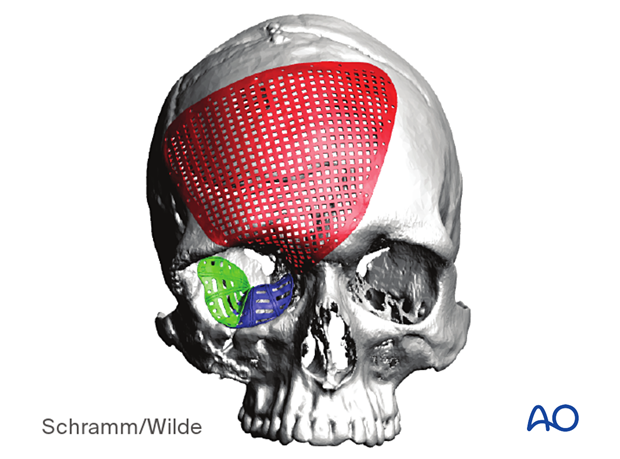
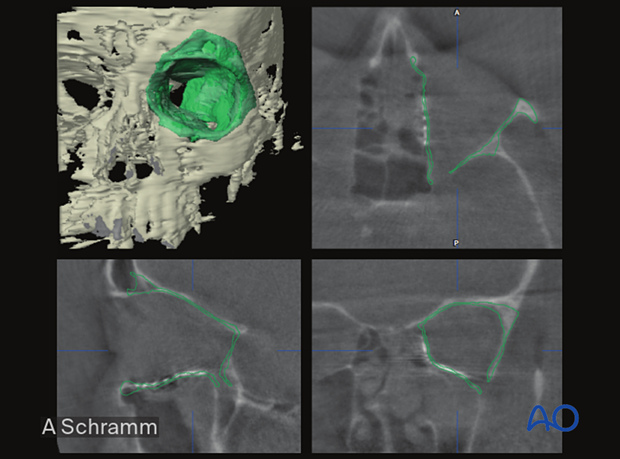
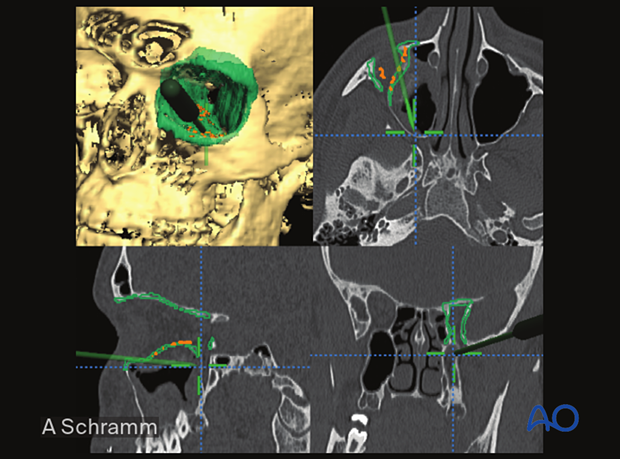
The illustration shows a patient-specific orbital implant used to reconstruct orbital wall defects.

The illustration shows patient-specific orbital implants used to restore the orbital volume in empty socket syndrome.
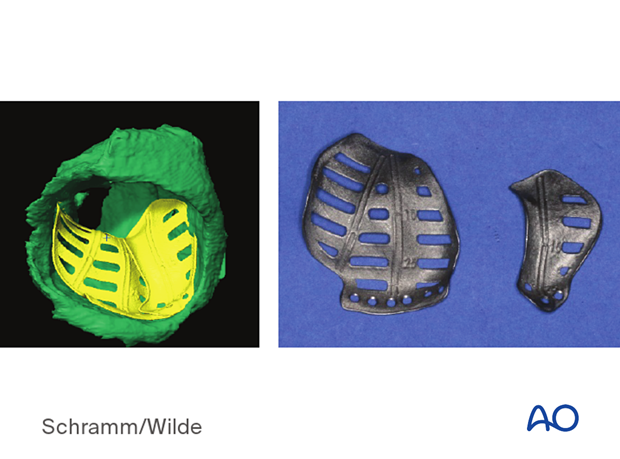
The illustration shows patient-specific implants used for mandibular alloplastic reconstruction.
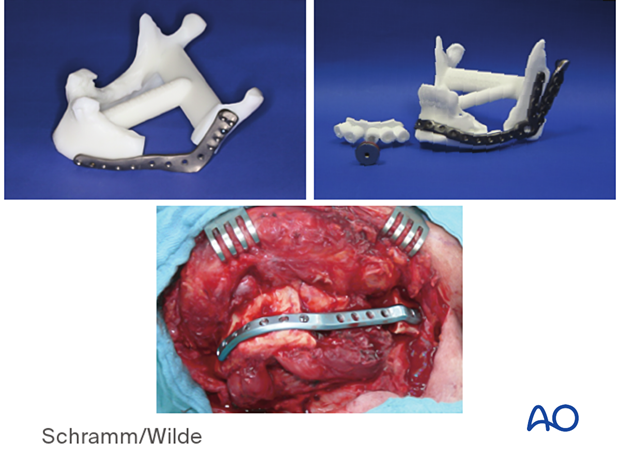
The illustration shows patient-specific implants used for TMJ reconstruction.
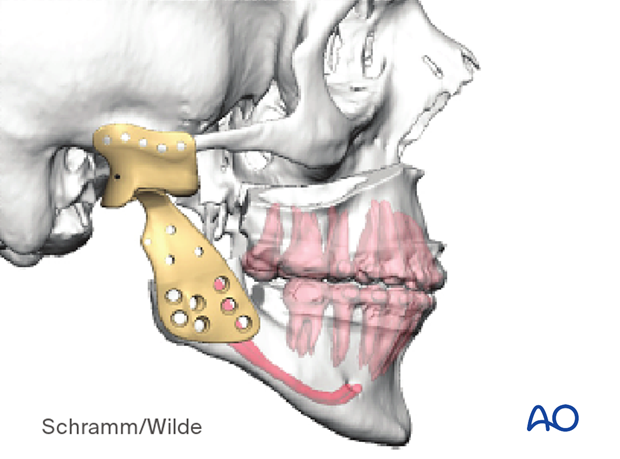
The illustration shows implants used for patient-specific prosthodontic reconstruction.
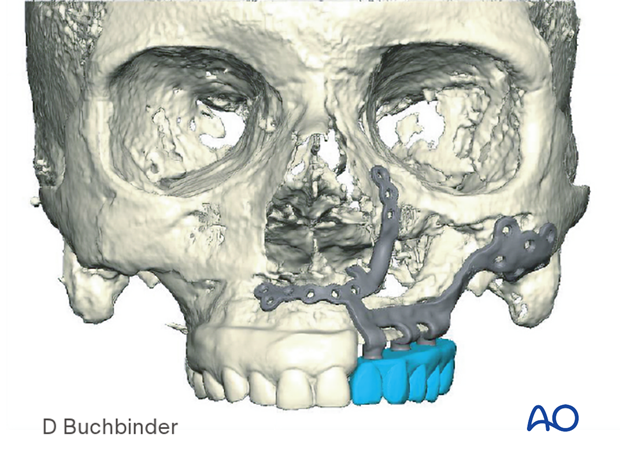
The illustration shows a patient-specific distractor.