Infection
1. Prevention of infection
All the following are important for avoiding infection after fracture surgery:
- Perioperative antibiotics
- Aseptic technique
- Soft-tissue care: preservation of blood supply, hemostasis, debridement, stabilization of fractures, tension-free wound closure
- Temperature control
- Nutritional support
For more information, read the full AO Surgery Reference chapter on prevention of infection.
2. Introduction
A fracture wound that becomes infected almost always results in prolonged treatment and a compromised outcome. A surgeon who treats fractures must be aware of factors which affect the risk of infection. Every effort should be made to reduce the risk of infection by following basic principles of fracture care.
Should infection develop, it is important that it is promptly recognized and effectively treated according to basic principles of patient care, surgery, and microbiology.
The factors which reduce a patient’s ability to resist infection should be corrected to the greatest extent possible. Necrotic or severely injured tissue and foreign materials should be removed surgically. Wounds should be closed as soon as their condition permits. Fractures should be stabilized surgically. As they are foreign bodies, internal fixation devices compromise host resistance in the event of infection, and must be removed unless they provide appropriate stability, and the infection responds promptly to treatment. Infecting microorganisms should be identified, their antibiotic sensitivities defined, and appropriate specific antibiotics administered as an adjunct to surgical treatment.
Fracture wounds almost always damage the surrounding soft tissue envelope, compromising its ability to heal and therefore increase the risk of infection.
3. Classifications of wound infection
Introduction
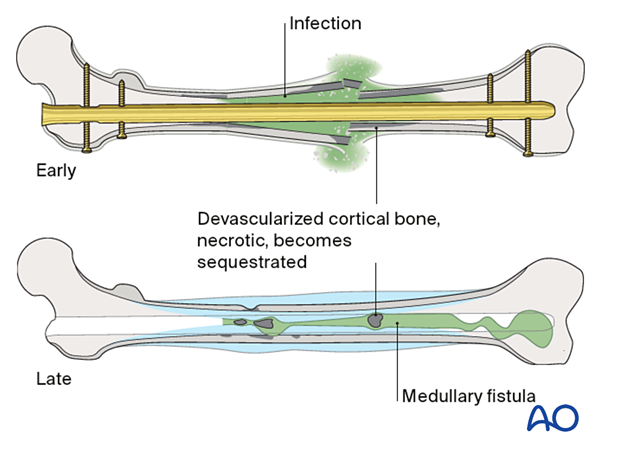
A variety of factors influence the prognosis of fracture wound infections. A fracture wound begins with damaged soft tissue and bone, and therefore has locally reduced resistance to infection. There may also be necrotic bone, and perhaps foreign material, where bacteria are protected from host defenses and blood-borne antibiotics, often sheltering in biofilms.
Factors to consider in classifying infections are:
- Duration of infection
- Anatomical area of bony involvement
- Host resistance (systemic and/or local)
- Status of fixation
- Fracture healing status
- Bacteriology
Classification according to chronology of onset of infection
Onset of fracture-site infection can be related to the time elapsed since the fracture occurred. Identification of infection may be delayed, beginning some time after the injury. This is relevant since progressive infection leads to infection spreading outward from the original infected site and causing additional tissue damage. It can be difficult to know exactly when an infection begins. When it is first diagnosed some time after the original injury, a careful history from the patient and/or review of laboratory data may suggest a shorter duration of active infection.
It is helpful to consider the major features of infection classified according to time after injury.
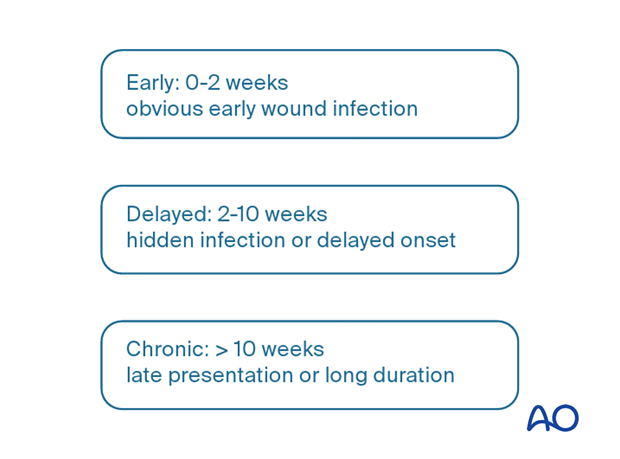
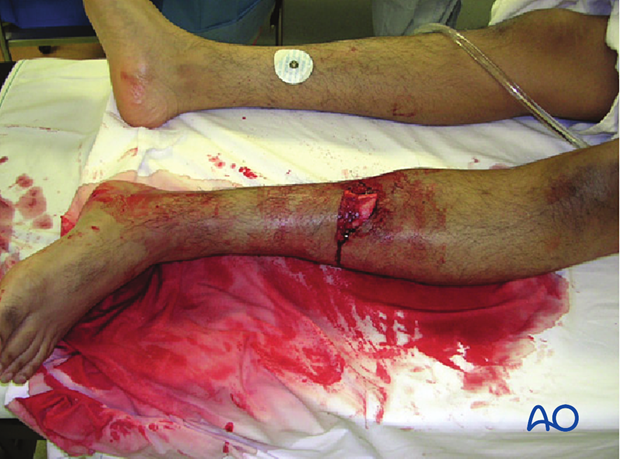
Early infection
Early infections generally occur less than two weeks after surgery. Their clinical signs are:
- Increasing local pain
- Redness
- Swelling
- Wound drainage
- Disturbed wound healing
- Fever
Infecting organisms are typically highly virulent and include Staphylococcus aureus, Gram negative bacilli and Clostridial infections (gas gangrene or tetanus).
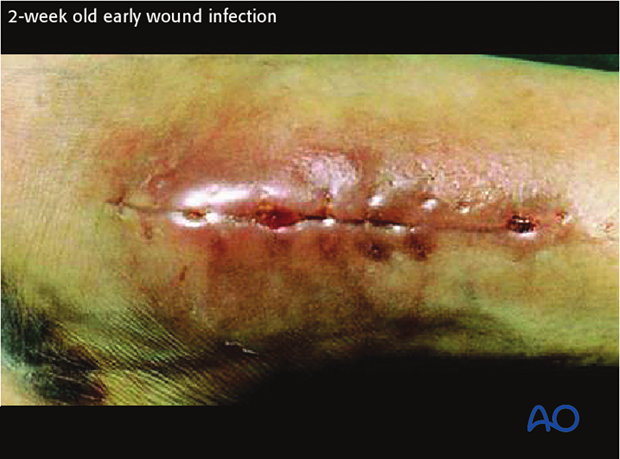
Early infections need prompt surgical treatment with debridement as well as appropriate adjunctive antibiotics.
Such infections are not “superficial”, as the entire fracture wound is almost always involved. Only surgical exploration allows comprehensive assessment and care.
In addition, tissue samples taken at this time offer the chance to culture organisms hidden in biofilms.
Surgeon neglect allows the infection to progress to a point where relatively simple debridement may not succeed.
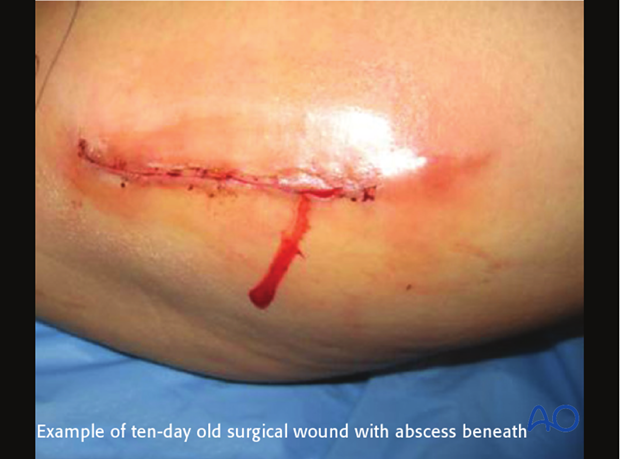
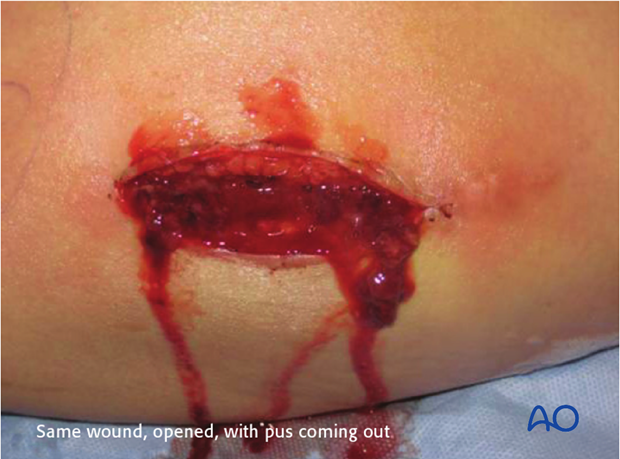
When symptoms have been present for less than two weeks and internal fixation implants still provide appropriate stability, it may be possible, after thorough debridement and with appropriate antibiotics, to retain them until the fracture is healed. Should the infection fail to resolve rapidly, repeated debridement, including implant removal with alternative fixation, will be necessary.
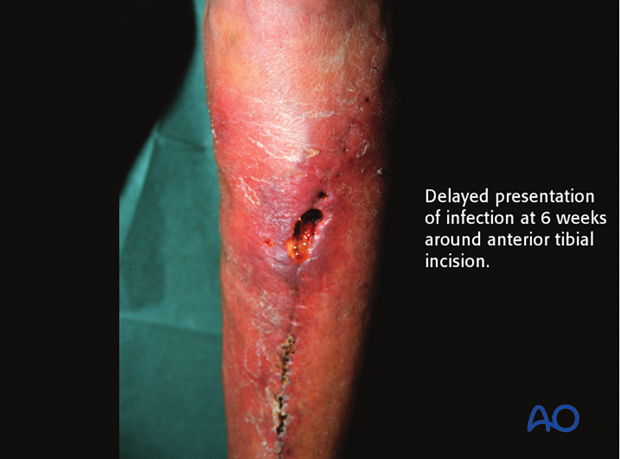
Delayed presentation of infection
In some cases, the presentation of infection is delayed and may not be seen until several weeks after surgery. More aggressive treatment is usually necessary in these instances.
More information is provided here about delayed presentation of infection.
Chronic infection
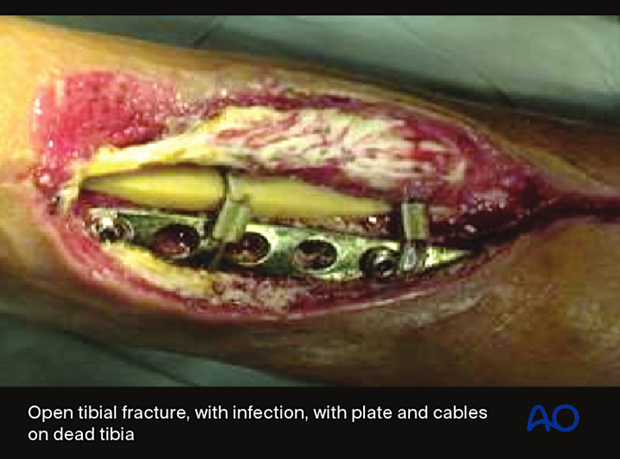
An infection which has been present in a fracture wound for more than several weeks is a serious and challenging problem. Typically, such wounds have failed previous treatment attempts, or have been neglected.
If internal fixation devices are present, they almost certainly contain microorganisms within a layer of adherent slime. These chronic fracture site infections also include dead bone and soft tissue. The necrotic tissue may be localized or diffuse. Without its complete removal, there is essentially no hope of controlling the infection. Furthermore, fracture healing will not occur until the infection is controlled, and tissue viability restored.
Treatment requires radical debridement, often involving several operations, and fracture stabilization, with either new external or internal fixation implants. Extensive bone removal may result in a segmental defect, or loss of an involved joint. In some cases, the extent of tissue removal requires amputation. Failure to control infection may also lead to loss of limb.

Recurrent osteomyelitis
Osteomyelitis may recur, sometimes years after the original infection appears to have been resolved. Treatment depends upon the following:
- Extent of fracture healing
- Status of the surrounding soft tissue envelope
- Implants in situ
- Sequestra
- The patient’s health status
More information is provided here about recurrent osteomyelitis.
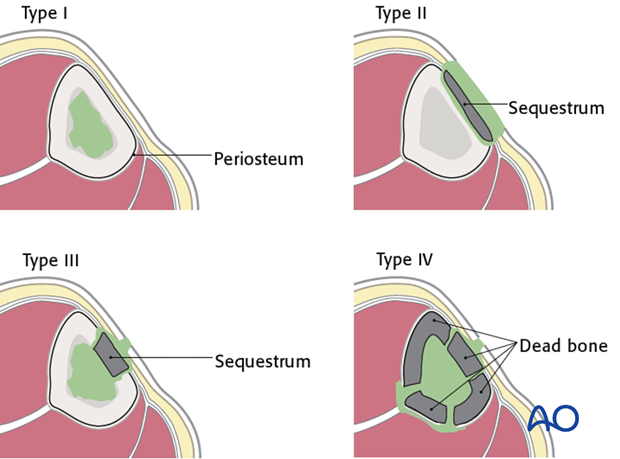
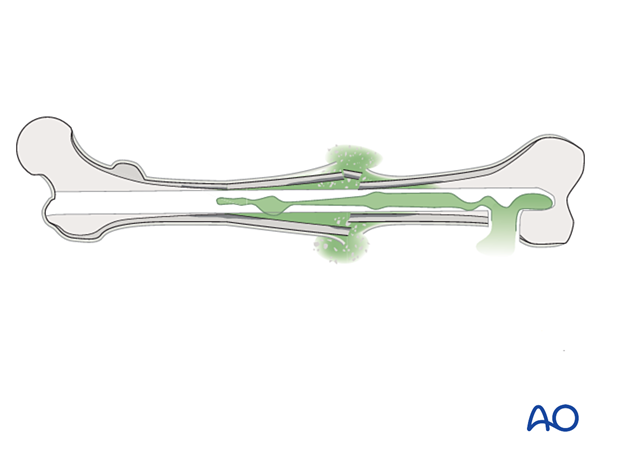
Cierny’s anatomical classification
The anatomical classification of osteomyelitis (see diagram) is important for understanding and localizing the infection.
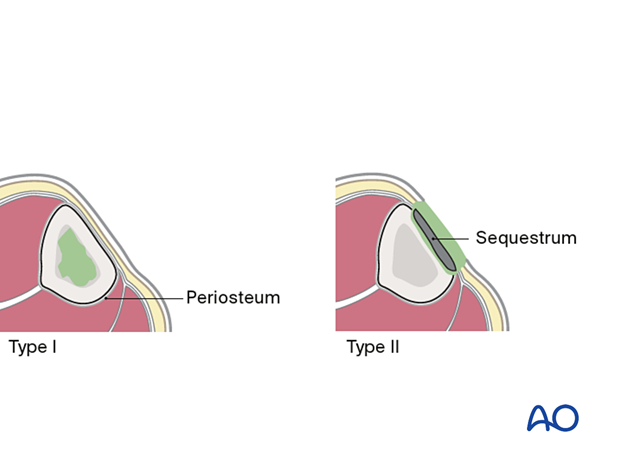
- Type I (medullary osteomyelitis) diffusely involves the intramedullary cavity, essentially always after medullary nailing. The entire medullary canal is involved and will require debridement (nail removal and reaming).
- Type II osteomyelitis involves the outer cortical area, the subcutaneous tissue, and the skin. The infection resides within an isolated area consisting of cortical sequestra and granulation tissue. Treatment involves debridement of the affected cortex and soft tissue envelope.
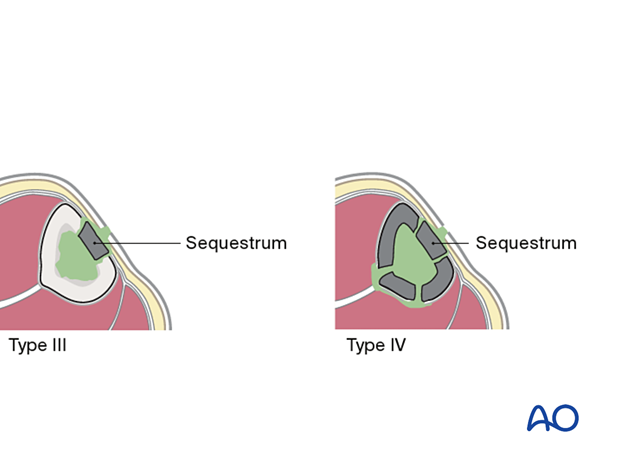
- Type III osteomyelitis (localized full-thickness cortical involvement) will require debridement of all necrotic bone. During debridement, the full extent of the necrotic area becomes evident. This may weaken the bone or produce significant dead space. Soft-tissue coverage may be inadequate and may require reconstruction. Fracture healing may be a problem that requires identification and treatment.
- Type IV osteomyelitis diffusely involves the entire circumference of at least a segment of the bone. Usually, the entire bone segment must be removed to eliminate necrotic tissue and persistent bacteria.
Host resistance to infection – Introduction
Patients vary in their ability to resist infection. In some cases, a patient’s response to infection is so impaired that all efforts at treatment fail. To understand the problem of posttraumatic infection, it is important to assess each patient’s immunocompetence.
Local factors are limited to the site of infection and include focal arterial insufficiency, venous stasis, previous radiation therapy, etc.
Systemic factors that impair host resistance include tobacco smoking, malnutrition, diabetes mellitus, renal failure, chronic steroid or immunosuppressant use, etc.
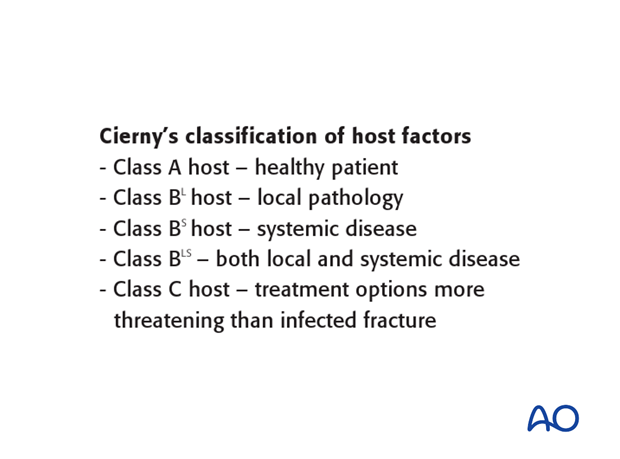
Cierny’s classification of host resistance to infection
Cierny has provided a classification scheme for host resistance in addition to that presented above for anatomical classification. Both local and systemic factors are involved. Cierny’s classification identifies the uncompromised patient as class A. If significant compromise is identified, the patient is class B, either based on local factors (B L), systemic factors (B S), or both (B L, S).
Class C patients are either so compromised that the risks of treatment outweigh the benefits, or the symptoms of their infection are sufficiently limited that surgical treatment is not indicated.
A class A host is a healthy patient. The infection is not the result of impaired systemic host resistance. It must be remembered that a very severe injury can result in a focal defect of host resistance, because of locally impaired vascularity and tissue damage. An otherwise healthy patient with this degree of persisting local damage belongs in class B (B L).
Class A hosts are generally able to tolerate any appropriate surgical procedure. Tissue response to infection is strong, soft-tissue viability is excellent, and healthy tissue is available for transfer, if needed. With or without long-term antibiotics, the host can recover from a completely resected infection.
A class B L host has locally compromised soft tissue, or bone. The patient’s injury or previous surgery may compromise tissue healing. For example, the patient may have had radiation, or previous injury to this region. Other potential local impairments are vascular disease or lymphedema, which impair wound healing and response to infection.
This particular patient’s localized ischemia may be correctable with arterial reconstruction. This may improve their resistance classification.
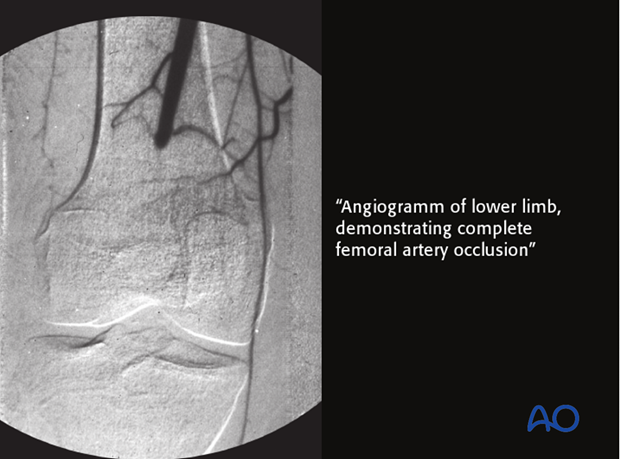
Many systemic diseases can affect a host, reducing the ability to fight infection. Extremes of age, malnutrition, and obesity are three very common causes of systemic host problems. Smoking, alcohol, steroids, etc. also compromise host resistance. Systemic diseases such as diabetes, and renal or liver failure, impair tissue response to infection.
With such patients, every effort must be made to identify and treat the systemic problems, but it is not always possible to restore normal host resistance. In practice, when dealing with an active infection, it is appropriate to begin treatment involving drainage, debridement, and appropriate antibiotics, with minimally invasive fracture stabilization (external fixation). Preferably, impaired host resistance can be improved before definitive surgical reconstruction. If this is not possible, treatment failure, and/or amputation become likely.
Class C patients are either so compromised that the risks of treatment outweigh the benefits, or the symptoms of their infection are sufficiently limited that surgical treatment is not indicated.
Bacteriology
One, or more, bacterial species may be present in fracture wound infections. The source can be the patient’s skin, scene of injury, or hospital contamination. If there are signs of infection, obtain multiple surgical (deep wound) specimens for culture and sensitivity testing.
More information is provided here about bacteriology.
Fracture union and fixation devices
Fracture infections are influenced by factors involving the fracture and its prior treatment. Infections interfere with fracture healing. Infections are harder to eradicate from nonunited fractures. Treatment plans must address a nonunion, if it exists, with decisions about bone resection, stabilization, grafting, etc.
Fracture fixation devices impair host resistance. If they provide absolute stability, this benefit may overcome their detrimental effects. Thus, in carefully selected cases (low-virulent organisms and healthy tissues), internal fixation may be retained, if stability is adequate.
If there is instability, or the infection does not resolve promptly, plates, or intramedullary nails should be removed and replaced with external fixation.
4. Risk factors for fracture wound infection
Introduction
Fracture wound severity may be the most important factor influencing risk of infection. An additional influence is the ability of the host to resist infection, based on both systemic and local factors. This has been discussed above in reference to classification of wound infections.
Wound-severity classification
Most research shows that infection rate increases with severity of soft-tissue injury. Bone injury is a less important factor, providing that injured bone has a blood supply.
The AO classification of fracture wound severity separately addresses Fracture, Integument (skin), Muscle, Nerve and Vascular injuries. A full understanding of wound severity requires consideration of each of these elements. Particularly important is the separation of closed and open fracture wounds. This is done in the integumentary (skin) classification. Open fractures have a notably higher risk of infection.
The AO classification supports the statement, “a fracture is a soft-tissue injury that happens to have a broken bone inside”.
Arterial injury
The commonly employed Gustilo-Anderson open-fracture classification separately identifies open fractures with arterial injuries that require repair to restore limb viability (classified as IIIC). Gustilo and others demonstrated a 50% risk of osteomyelitis after such injuries, with amputation a frequent outcome.
Gustilo RB, Mendoza RM, Williams DN Problems in the management of type III (severe) open fractures. A new classification of type III open fractures. J Trauma 1984 Aug;24(8):742–6.
5. Diagnosis of wound infection
Introduction
Early identification of a wound infection is the first step towards prompt treatment, which is a requirement for optimal results. Any fracture wound, whether caused by the injury, or created by the surgeon, is at risk of becoming infected. Recognition of those factors which predispose to infection may increase the surgeon’s alertness to the possibility of infection. Early signs of infection are not specific and may easily be misinterpreted. Inflammation is normally present in the region of a fracture, even without infection. Often, the first sign of wound infection is that the inflammation does not resolve normally. Certainly, increasing signs of inflammation (wound drainage, redness, swelling, pain and tenderness, fever) must be regarded as a strong indication of possible infection.
Should the surgeon be concerned about infection, efforts to diagnose or exclude this possibility must be undertaken without delay.
Evaluation of possible infection
Presence of bacteria within an inflammatory wound exudate is definitive proof of infection. Often this is most readily obtained by sterile aspiration of the wound, or by exploring it surgically. Microscopic examination (Gram stain) of the exudate, and appropriate bacteriologic cultures, provide evidence of bacterial presence. Most fracture infections are due to readily evident bacteria, although previous antibiotic treatment may interfere with microbiology studies. It must be borne in mind, additionally, that there may be few planktonic bacteria in the wound, and that most bacteria may be trapped in biofilms. Occasionally, increasing inflammation is evident without recoverable bacteria. While occult infection may be the cause, the surgeon should remember that a non-infectious cause of increasing inflammation is mechanical failure of fracture fixation. This may also co-exist with infection.
Systemic signs of inflammation, often associated with infection, can be identified by the following laboratory studies. By themselves, none of these prove nor exclude infection:
- Serial wound examinations
- Maximal daily temperature
- Complete blood count (CBC) with differential white cell count
- Erythrocyte sedimentation rate (ESR)
- C-reactive protein (CRP)
- Imaging: Plain x-rays, Computed tomography (CT) scan, Magnetic resonance imaging (MRI), Bone scan
- Bacteriology (Gram stain, culture, and sensitivity)
Classification of infection
An important guide to treatment is provided by classifying an infection according to several parameters:
- Duration
- Anatomical location
- Status of fixation
- Infecting organism(s)
- Viability of bone and soft tissue
- Host resistance
Optimal management of an infected fracture requires consideration of each of these factors. Each one must therefore be evaluated.
Clinical exam
Clinical symptoms and signs are most important for identifying the presence of infection. Increasing pain, drainage (either purulent or serosanguineous), swelling, redness, warmth, and tenderness all suggest the possibility of a wound infection. Progressive worsening of one or more of these findings is confirmatory. Thus, serial examinations may be required. While some inflammation is caused by a fracture, its severity should progressively decrease. Local infection must not be mistaken for normal postoperative inflammatory signs.

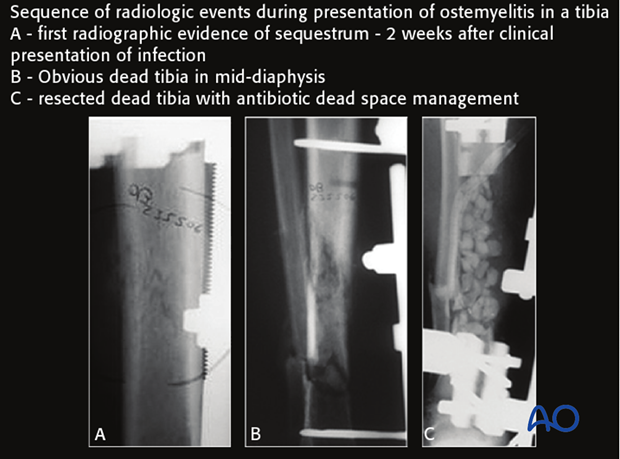
Radiographic investigations
In early infections, x-ray imaging procedures typically play a minor role. Radiographic findings of infection are usually not evident until at least two weeks after the onset of infection, even though bone involvement has already occurred.
Imaging becomes important in the later stages of infection. It is helpful to examine serial x-rays for progressive changes that suggest infection.
Radiographic signs are neither sensitive nor specific for infection. For example, radiographic evidence of implant loosening may be present with instability, infection, or both.
Ultrasound is useful for identifying accumulation of fluid (hidden abscess). This method is non-invasive, and may reach deeper layers, especially in the thigh. Ultrasound can also be helpful for guiding diagnostic needle aspiration.
Computed tomography better demonstrates differences in bone density, such as the dense white necrotic sequestrum illustrated here. It also offers a cross-sectional guide for exploration and debridement, particularly for bone fragments.
Although better than plain radiographs, CT scans do not have a high sensitivity or specificity in diagnosing infection.
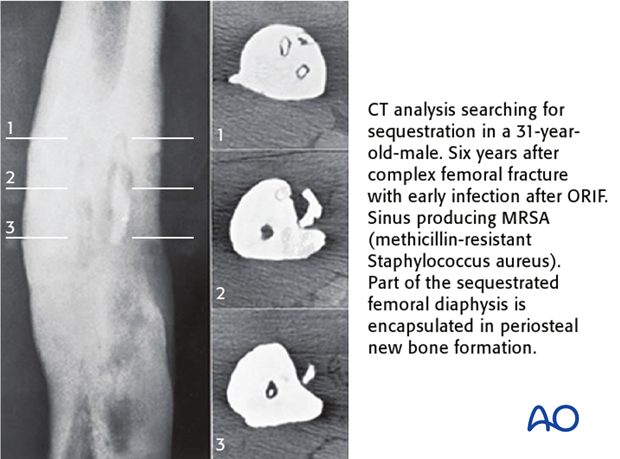
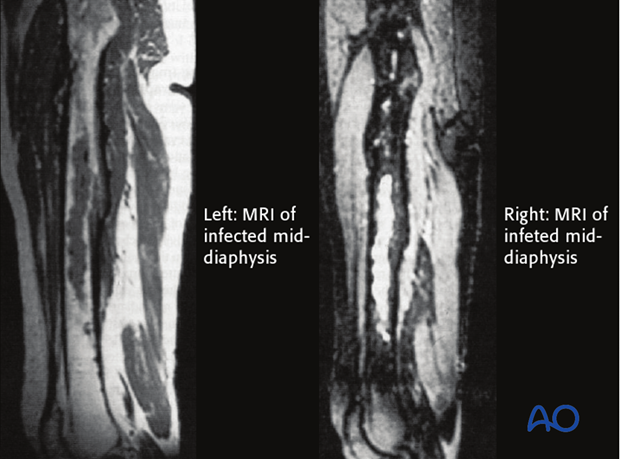
MRI presents an improved resolution of soft-tissue abnormalities and shows greater anatomic detail than other imaging studies. Nevertheless, artifacts created by metal implants can compromise image quality. Overall, MRI is much more sensitive and specific in identifying infection than x-rays and CT scans.
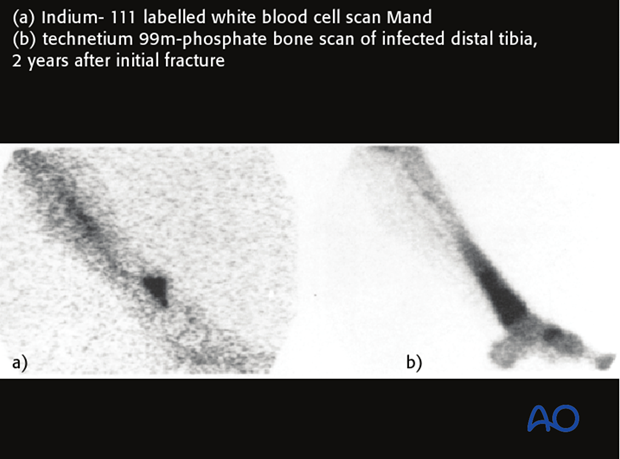
Uptake of technetium-labeled phosphate compounds (Tcm-MDP) is increased in areas of high vascularity, including infections and bone healing. With infection, a three-phase bone scan shows increased uptake of labeling in all three scan phases.
Absence of uptake suggests impaired vascularity or bone necrosis. Technetium bone scans detect increased bone remodeling which is present around all fractures for 12–24 months. Technetium bone scans cannot differentiate aseptic hardware loosening from infection. They have little value in assisting in the diagnosis of infection in the early postoperative healing period of acute fractures.
Indium-labeled white blood cell scans are much more specific in identifying inflammation and infection. The illustration shows a larger area of labeling with the Tcm-MDP scan than with the indium white blood cell technique. False positives and false negatives can still occur in instances of non-unions.
Bacteriology
Fluid can be aspirated sterilely preoperatively and sent for culture and Gram stain. Tissue specimens should be sampled from three or more suspicious sites, with two pieces of tissue from each site (one for microbiology and one for histopathology). Both aerobic and anaerobic cultures should be undertaken.
PCR bacterial identification, if available, is a more rapid and reliable technique than standard cultures.
Histological investigation can reveal a bacteriological etiology even if the bacteriological tests are negative. Superficial wound swabs should be avoided because of low sensitivity and frequent contamination by bacteria not responsible for the infection. Prior to tissue-sampling for culture, it is important to discontinue any antibiotic therapy for at least a week or two.

Septic arthritis
If septic arthritis is suspected, joint aspiration should be performed to evaluate the affected joint. If the white blood cell count is greater than 50,000, the joint is likely infected. Infection may exist with lower white blood cell counts, especially if the aspirate has positive growth. If infection is present, arthroscopic, or open irrigation and debridement with synovectomy must be performed. If significant cartilage degradation or surrounding osteomyelitis is identified, the implantation of an articulating antibiotic arthroplasty may be indicated.
6. Treatment of fracture wound infection
Antibiotics for wound infections
Initial treatmentBefore starting antibiotics, it is important to obtain adequate cultures. Several samples should be obtained in the operating room from different locations within the wound. Only if the patient is systemically septic should one consider providing antibiotics prior to wound exploration.
Selection of antibiotics for initial treatment is based upon the antibiotic sensitivities of likely infecting organisms, including a history of previously positive cultures for recurrent infections.
Generally, broad-spectrum, intravenous antibiotics are advisable as soon as cultures have been obtained. Initial antibiotics are selected, based on results of Gram stain and institutional frequency statistics (see table on left, Trampuz A, Zimmerli W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 2006 May;37 Suppl 2:S59-66).
Methicillin-sensitive Staphylococcus aureus is still the most likely infecting organism in most areas. Institutionalized patients have a higher risk of methicillin-resistant Staphylococcus aureus (MRSA) so that vancomycin may be advisable.
Gram negative coverage is wise for hospital-acquired infections. Ask about allergy history and modify antibiotic selection as necessary.
All of the above should be considered provisional treatment until culture results are returned. Consultation with an infectious disease specialist can be very helpful in selecting appropriate antibiotics, their doses, and interpreting cultures.
Once culture results and sensitivities are known, it is possible to choose the optimal antibiotic for management of a fracture wound infection. Remember that antibiotics are adjunctive to adequate debridement and recognize that an infection that does not respond to post-operative antibiotics may need repeat surgical exploration and debridement. Once again, antibiotic selection is aided by consultation with an infectious disease specialist or internist with interest and experience in antibiotic management. This is particularly true when dealing with resistant organisms and patients with drug allergies or sensitivities.
Prolonged intravenous antibiotics (eg, six weeks), use of combinations of drugs, and continued oral medication after initial intravenous therapy all need to be considered.
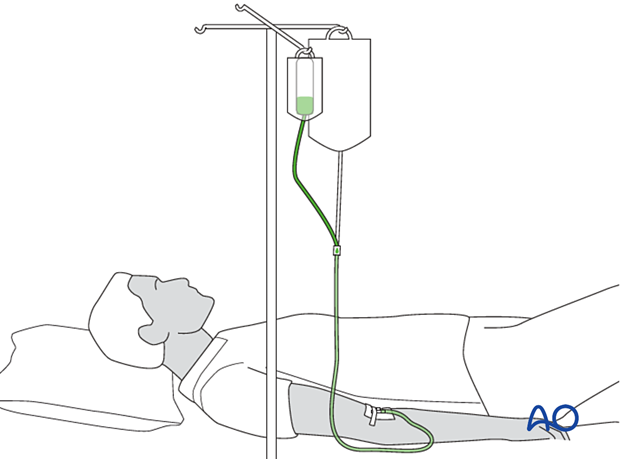
Antibiotic beads
Locally administered antibiotics may have a supplementary role in the management of musculoskeletal infections. Particularly when the infected wound has poorly perfused areas, or “dead space”, antibiotic-laden cement is frequently used, both to fill the space and to deliver high doses of local antibiotic with low risk of systemic toxicity. A common technique is the use of antibiotic-laden polymethylmethacrylate (PMMA) beads.
Antibiotic-impregnated beads may be purchased in some countries (although they can be expensive) or made by the surgeon at a lower cost. One gram of cefazolin (a first-generation cephalosporin) or vancomycin is cheap and accessible. It is mixed with one standard package of PMMA and, as it hardens, beads of 5 mm are carefully wrapped around a non-absorbable heavy nylon stitch (see intraoperative photograph).
Tobramycin powder (1.2 g) may also be mixed with PMMA for broader-spectrum coverage.
Initial surgery for fracture site infection
If an infection is strongly suspected, the surgeon should proceed with exploration of the wound, obtaining tissue for culture, and removing all non-viable tissue and exudates.
Debridement (see below) involves the surgical excision of necrotic and/or infected tissue from the wound.
Tissues to be removed include:
- Exudate (hematoma and pus)
- Unhealthy wound margins
- Abscess membrane
- Sinus tracts
- Granulation tissue
- Dead bone, including sequestra
Look carefully for dead bone and any remaining foreign material. Preserve nerves and blood vessels and viable tendons. With acute wound infections, internal fixation devices may be retained, if they provide sufficient mechanical stability and are unlikely to have a significant volume of adherent bacteria. With more-established infections, remove all local hardware and provide stability with an external fixator or new internal fixation device.
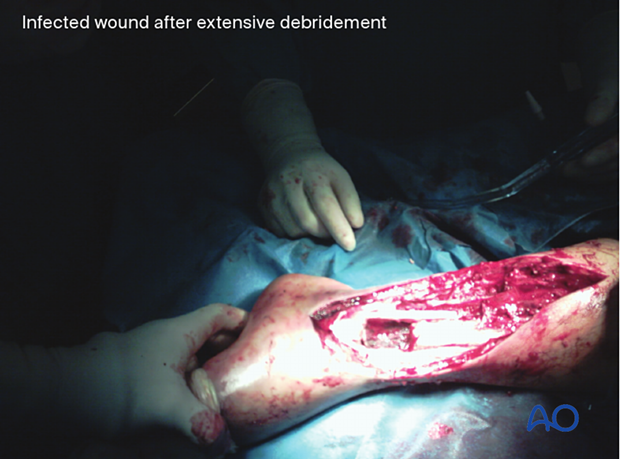
Debridement
The surgical excision must be complete and thorough.
Adequate, timely debridement is the most important element of the treatment of a fracture-site infection.
With well-established infections, there may not be a clear demarcation between viable and non-viable tissue. Radical excision may be necessary to eliminate infection, even though it may increase the complexity of reconstruction.
After debridement, the surgical site should be thoroughly irrigated to reduce the bacterial population. In cases with substantial amounts of dead tissue, or grossly purulent wounds, repeated surgical debridement is indicated. Deciding what tissue to remove and what tissue to retain is the essential challenge of debridement. Such decision-making is learned from experienced surgeons and from practice. Common errors are failure to remove enough compromised tissue, and/or doing so in a way that injures the retained tissue. An organized approach that proceeds in orderly steps through tissue levels is required. Any non-viable skin is excised. The incision should be extended, as necessary, for adequate exposure of the whole infected zone. The depths of the wound are then exposed and must include all of the previous surgical exposure. Any extension of hematoma, pus, or necrotic tissue, should be explored fully.
With acute wound infections, internal fixation devices may be retained, if they continue to provide appropriate mechanical stability. If the implants are loose, and in more established infections, it is advisable to remove all hardware and to restabilize with an external fixator or new internal fixation device.
Excise the surface of the exposed tissue to leave clearly viable margins of subcutaneous tissue, fascia and muscle. Non-viable bone must also be excised.
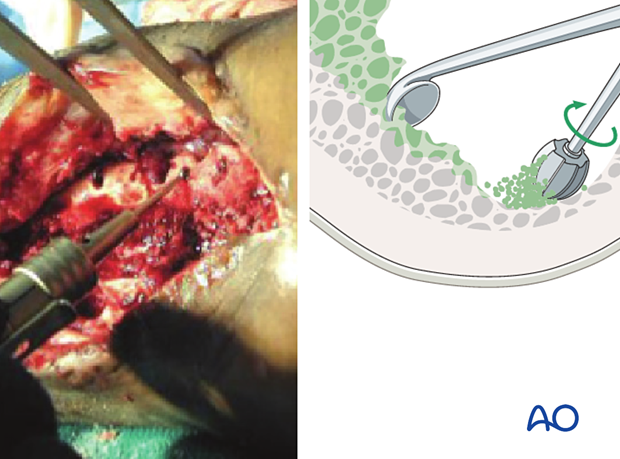
In areas where dead bone exists, remove with a high-speed burr or osteotomes until bone bleeding is encountered. Small bleeding osseous vessels (“paprika sign”) indicate viable bone.
Abundant irrigation helps to remove bacteria, pieces of dead tissue, and blood clot, and improves the surgeon’s ability to examine the wound.
A “second look” and possibly further debridement should be considered until the wound is completely viable. Staged debridement is illustrated to the left.
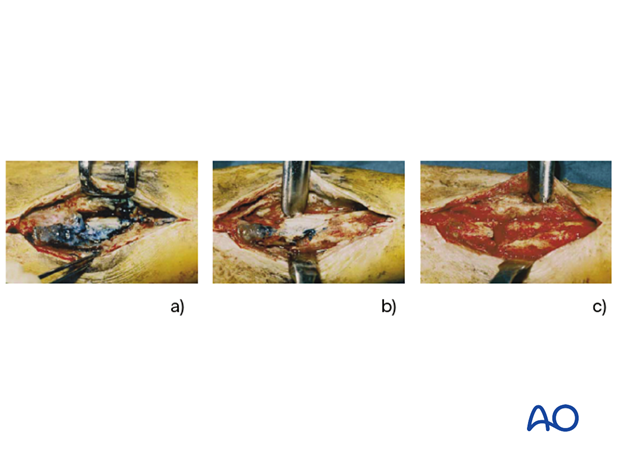
Management of an infected wound
After the first debridement of an infected fracture wound, it is usually wisest to defer primary closure. Definitive closure may be considered when final debridement has been completed.
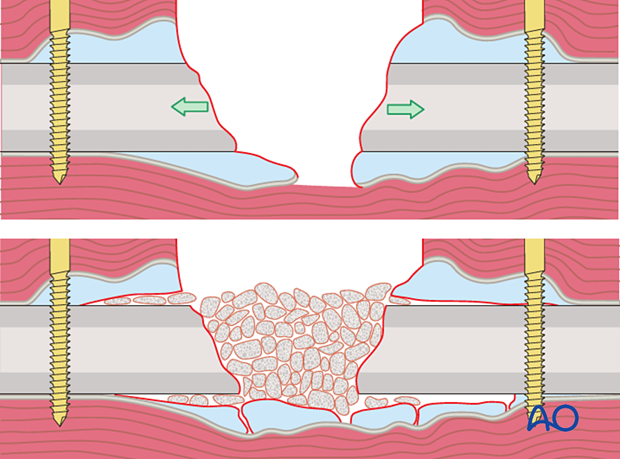
Empty space (“dead space”) under tissue flaps, or within bony cavities, within the debrided wound allows re-accumulation of exudate and may become a reservoir of bacteria. Antibiotic levels may be low in this poorly perfused fluid accumulation. Filling this dead space in some way is an important part of wound management.
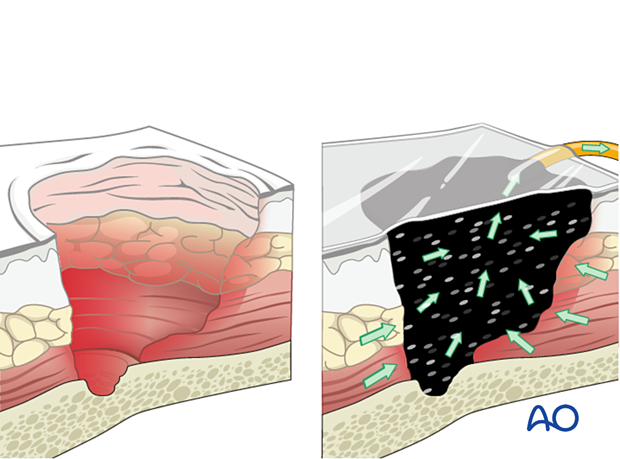
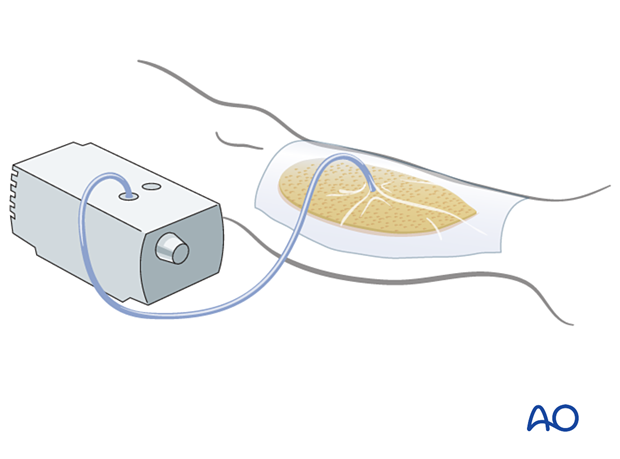
Treatment strategies for filling dead space include antibiotic beads or other temporary space fillers (calcium sulphate, bone cement, etc.). The wound itself should be covered to avoid desiccation or contamination. This can be done with an impermeable dressing (adherent plastic), or vacuum-assisted closure device, as illustrated. The latter, by applying sub-atmospheric pressure, reduces dead space volume in pliable tissues.
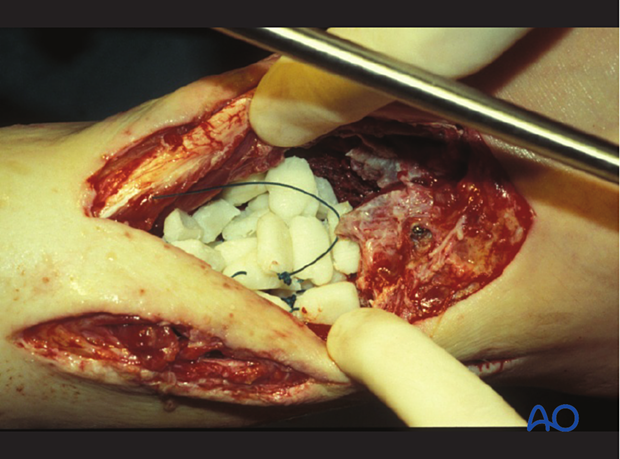
Dead space in bone
A significant bone defect may be filled with antibiotic beads, or other space filler. The technique outlined by Masquelet is effective (Masquelet, AC, Begue, T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010 Jan;41(1):27-37).
This involves insertion of a cement spacer that contains antibiotics, which is left in place for four to six weeks while a membrane develops around it. Then the spacer is removed, and the surrounding osteogenic membrane is filled with autologous bone graft. This often consolidates to fill small to medium-size defects. This membrane does not form satisfactorily around cement beads and is destroyed during their removal.
Implant retention or removal: plates and screws
As long as a plate and screws provide absolute stability, fracture healing can usually take place despite the presence of infection undergoing treatment. Fracture stability is more important than the negative effects of a metallic foreign body upon host defense.
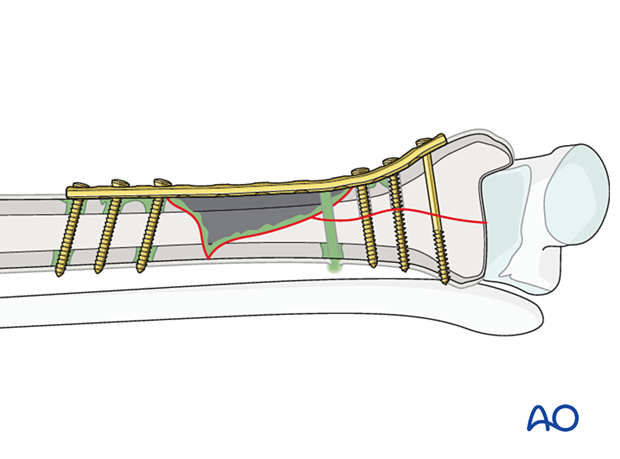
Typically, fixation remains stable in early infections (up to six weeks or so). If implants become loose at any time, they must be removed. External fixation stabilizes the fracture and avoids positioning a metallic foreign body in the infected wound. This is often the best choice for early fixation after infection. Only if the soft tissue envelope allows can the use of a new internal fixation construct be considered.
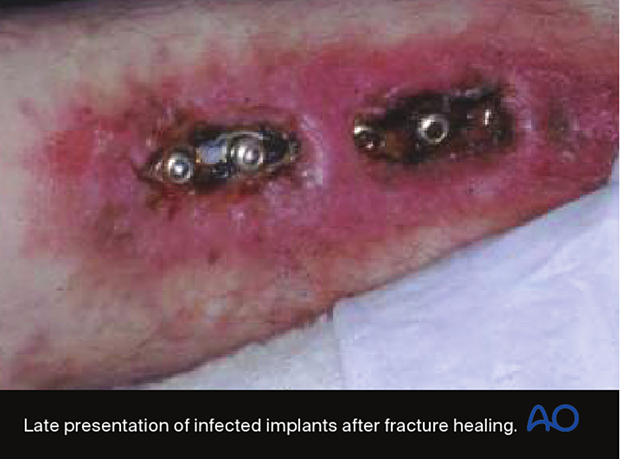
If an infection is identified and treated early, and the fixation remains stable, with appropriate debridement and antibiotics, the infection will typically respond and remain suppressed during fracture healing. Despite successful fracture healing, recurrent infection often ultimately results in hardware removal.
After the “infected hardware” is removed from the united fracture, and the wound debrided, the infection usually resolves satisfactorily with a low risk of recurrence.
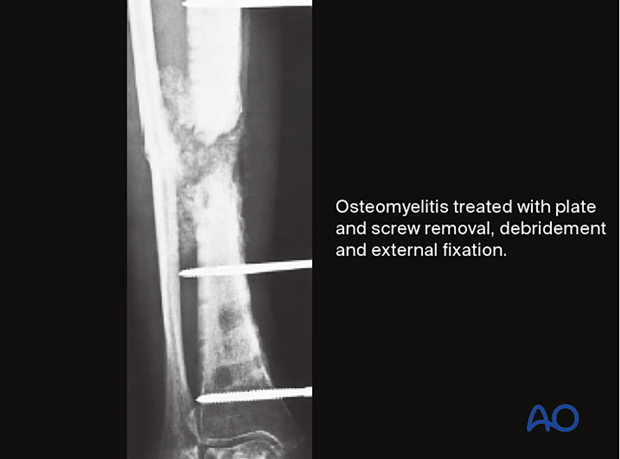
Implant retention or removal: IM nails
If a fracture site infection develops after intramedullary (IM) nailing, it is likely that the infection has spread along the medullary cavity. The infection may be early or late, before or after union. Adequate debridement requires removal of the nail and reaming of the medullary canal.
During initial clearance, a distal opening can be created at the lower end of the nail track, to allow debris from reaming to escape, and to drain the intramedullary canal. The canal is then reamed to a diameter 1.5 mm larger than the removed nail and is thoroughly and copiously washed out. A reamer-irrigator-aspirator device is ideal in managing intramedullary infections.
Any obvious cortical sequestra need to be removed in an open procedure.
An external fixator should be applied for stability if the fracture is not healed.
If the infection is early, and due to a less virulent bacterium, then renailing might be considered.
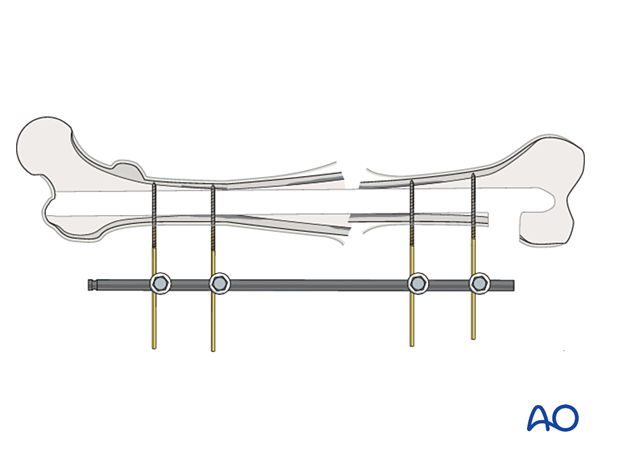
If the infection is late, or due to resistant organisms, external fixation may be preferable. An alternative is to temporarily place a reinforced, antibiotic-containing polymethylmethacrylate (PMMA) nail into the medullary canal. This is done after reaming out the infective membrane and undertaking a thorough lavage of the IM canal. Excision of any sinus track and sequestra must also be carried out.
An antibiotic-loaded PMMA nail is prepared by injecting liquid bone cement, pre-mixed with antibiotics (eg, tobramycin 1 g per cement batch) into both ends of an appropriately sized chest tube which is vented in the middle to allow complete filling. A small-diameter flexible rod (eg, nailing guide wire) is inserted before the cement hardens. The chest tube is then cut off.
The “nail” can be left in situ until the fracture has healed, or until the infection is under control, and then replaced with a solid metallic nail. A solid nail is used rather than a hollow nail because a hollow nail may become a hiding place for bacteria.
A conventional nail that is covered with antibiotic bone cement can also be used. The benefit of this type of nail is that the proximal and distal ends can be affixed with interlocking screws, allowing control of length, alignment, and rotation. In addition, weight bearing can be allowed.
Implant retention or removal: external fixators
Since external fixators are usually distant from the fracture wound, their removal is rarely required, but adjustment, or reinforcement, may be needed to ensure alignment and stability.
Should an external fixator pin track become infected, the pin should be removed, the pin site debrided, and a new fixation pin placed through clean tissue. A common mistake is not carrying out a thorough debridement of such pin tracks. This may be done most easily with a drill bit that is slightly larger than the residual pin hole. Inadequate pin-site care may result in persistent infection.
Temporary stabilization
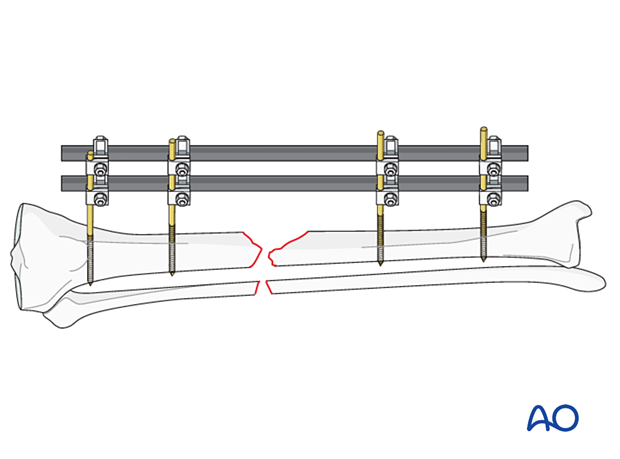
If a fracture has become infected, it requires debridement and fixation with either an external or internal fixator. The illustration on the left demonstrates proper external fixation placement.
The details of external fixation must include planning for wound care and preserving access for flap coverage. Additionally, fixator configuration should ensure excellent stability.
An external fixator can be used as a temporary, or a definitive stabilizer. Even if external or internal fixation will be used definitively, it may be best to use a temporary fixator after initial debridement. This aids repeated debridement and wound care. Once further wound access is unnecessary, a new fixation construct that provides optimal stability can be applied.
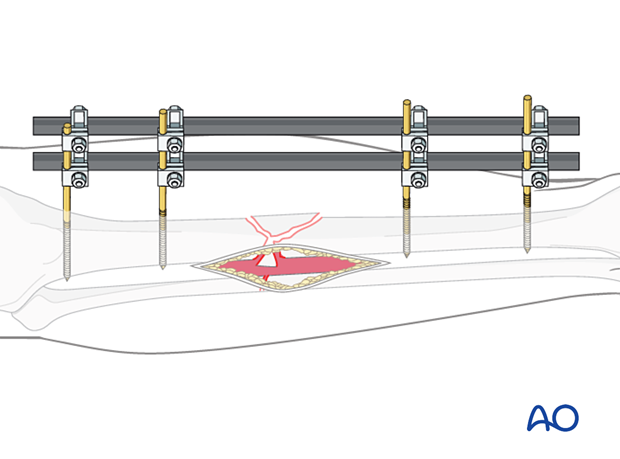
Avoidance of pin complications
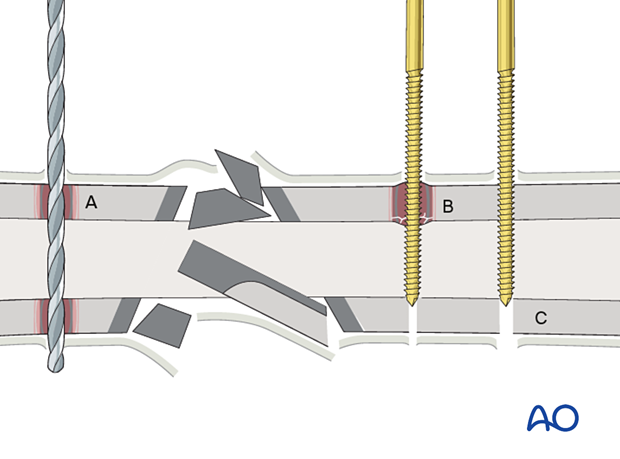
This illustration shows the results of incorrect and correct insertion of screws/pins for external fixation. A complex fracture caused by direct trauma is shown.
- Drilling at excessively high speeds, or with blunt drill bits, produces heat necrosis in cortical bone.
- Insertion of Schanz screws or Steinmann pins with no or inadequate predrilling produces considerable heat and small, necrotic fragments, or ring sequestra.
- Correct predrilling and placement of Schanz screws or Steinmann pins minimizes the risk of pin-track osteomyelitis.
Wound closure
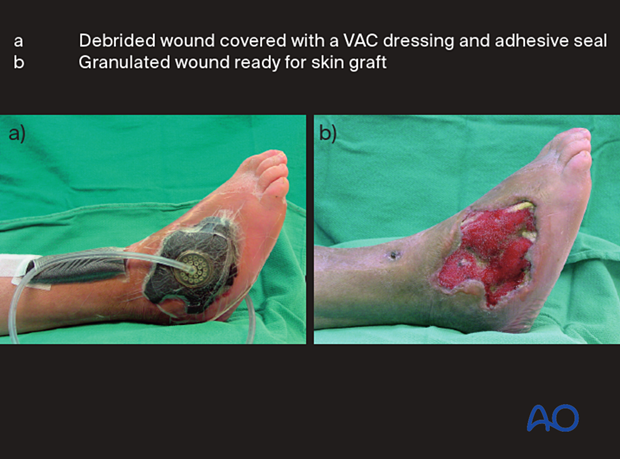
After debridement has been satisfactorily completed, in one or more procedures, consideration must be given to choosing the best means of wound closure. A tight suture closure risks wound healing problems. If primary closure is chosen, the application of an incisional wound vacuum-assisted closure (VAC) can be beneficial. Even if used, the surgeon must watch for wound breakdown or recurrent infection. Another alternative is to leave the wound open and allow it to heal by secondary intention with a VAC or with subsequent split-thickness skin grafting. This is more appropriate for a wound without exposed hardware, bone, tendon or neurovascular structures.
A VAC can be used to reduce the size of an open wound and promote granulation tissue which may accept a split-thickness skin graft. An alternative temporary dressing for an open wound is an impermeable adhesive drape to create a space (bead pouch) for antibiotic beads. A clean granulating wound with a healthy viable base may be subsequently closed with a split-thickness skin graft.
If the wound cannot be closed primarily or cannot be safely covered with granulation tissue from a wound VAC or split-thickness skin grafting, some form of flap coverage will probably be necessary.
Closure with local or free flaps is typically appropriate for larger and more complicated wounds, once they have been adequately debrided.
It is important to close a complex wound promptly rather than to leave it open and risk superinfection.
The example given here shows a soft-tissue and bony defect in the anterolateral aspect of the distal tibia. A locked intramedullary tibial nail is visible in the depths of the defect.
To cover this defect, a soleus flap is transposed. The pedicle of the flap must be surrounded by an adequate soft-tissue envelope, in order to ensure good venous drainage. This also helps to prevent kinking of the vessels. The gastrocnemius is preserved in the bed of the donor defect.
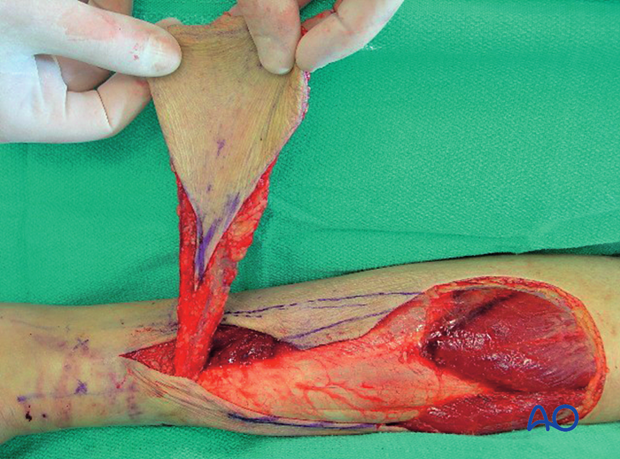
Soleus flap covering the defect.
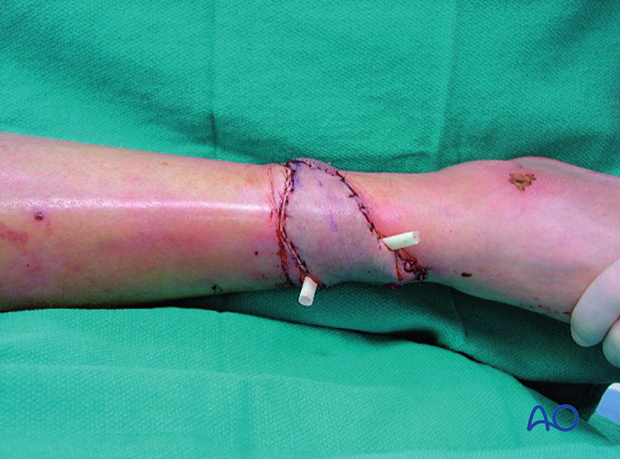
Filling dead space
Debridement of soft tissue or bone may leave dead space between bone ends or under flaps. If present, dead space should be filled, usually with a material that delivers high levels of antibiotics. This can be done with a block of antibiotic bone cement or bone cement beads. This illustration shows antibiotic beads placed within potential dead space under a muscle flap. If this is done, plans should be made for later exposure and removal of beads. Beads are usually replaced with bone graft.

Definitive stabilization
The anatomical classification of adult osteomyelitis helps determine definitive stabilization:
- Medullary osteomyelitis (Type I) is associated with an intramedullary nail which may or may not have been removed during initial surgery for infection. If the tissue is healthy, the bacteria are sensitive, and the patient is systemically healthy, it may be appropriate to stabilize the fracture with another nail.
- Superficial osteomyelitis (Type II) is typical of a healed fracture with superficial bone involvement, often under a plate. After removal of the plate, this superficial necrotic bone is removed with a burr, or hand instruments, until bleeding bone is seen. Fixation is replaced only if the fracture has not sufficiently healed. Wound closure may often follow soon after such superficial debridement.
- Localized, full-thickness osteomyelitis (Type III) may be associated with a non-united fracture. Although debridement must be performed to bleeding bone edges, enough cortical bone may be resected that the structural integrity of the bone is compromised. A subsequent fracture might result. Mechanical protection will be necessary, typically with an external fixator. More complex options for soft-tissue closure (local or free flaps) may be required. In some cases, plates or nails will be chosen for fixation. This hardware should not be applied until wound coverage can be carried out. Similarly, bone grafting is often necessary, and this should be delayed until coverage is obtained.
- Diffuse osteomyelitis (Type IV) has widespread areas of infected and/or necrotic bone, so extensive debridement is necessary. This may result in a segmental defect. Such defects will require bone grafting, bone transport, or fixing in a shortened position if loss of length is acceptable. Wounds associated with type IV posttraumatic osteomyelitis often need complex flaps for closure. Such widespread infection may require amputation for successful treatment.
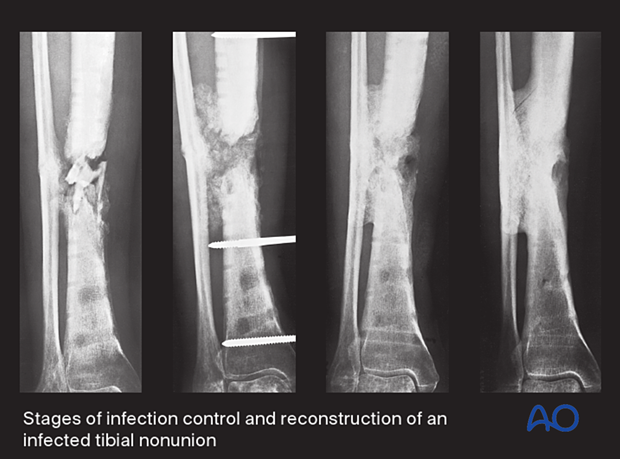
Infection control before bone reconstruction
Elements of infection control:
- Identification of the infecting organism/s
- Eradication of the infection by surgery combined with appropriate antibiotics and systemic support
- Creation of a viable and stable bone and soft-tissue envelope with either an external or internal fixator
- Skeletal reconstruction comes next:
- Consider likely reconstructive options (bone graft, transport, even amputation)
- Preserve options (eg, by incision placement and/or external fixator pin locations) if doing so does not compromise infection control
Stabilization
Skeletal stability assists the following:
- Enhanced wound care
- Eradication of infection
- Fracture healing
- Final reconstructive surgery (soft tissue and bone)
- Functional aftercare
External fixation is a means of stabilization in infected fractures and nonunions. It provides stability without the need for implants inside the infected wounds.
In septic surgery, external fixation may need to remain for up to a year, or more. It may be necessary to replace one or more pins during the course of treatment. There are three basic systems:
- External fixation with Schanz pins and tubular frames
- The ring fixator anchored with tensioned wires
- Hybrid ring fixators (anchored with both tensioned wires and Schanz pins)
All three systems have advantages and disadvantages. External fixation with Schanz pins is the simplest option and is very adaptable. The ring fixator is the most complicated system but can be used for compression and lengthening. A hybrid frame is simpler than a ring fixator and tensioned wires may have fewer pin-related problems.
These fixators must provide stability as in any other fracture fixation situation. If there is a problem with stability (loosening or infection) then the fixator must be replaced.
The use of plates and nails will mean that metallic implants are present at the site of infection. This may compromise successful treatment of infection. These implants should therefore be used with caution in an infected wound. In some cases, with early, or mild, infection, these internal fixation devices may be retained. In most cases they should be removed and temporarily replaced with external fixation. Then, once infection is completely resolved, plates or nails may be considered for reconstruction of the now-uninfected fracture site. As with external fixation, any retained plates, or nails, must provide stability. If there is any question of instability because of loosening, or poor bone contact, then the fixation must be revised.
7. Fracture reconstruction
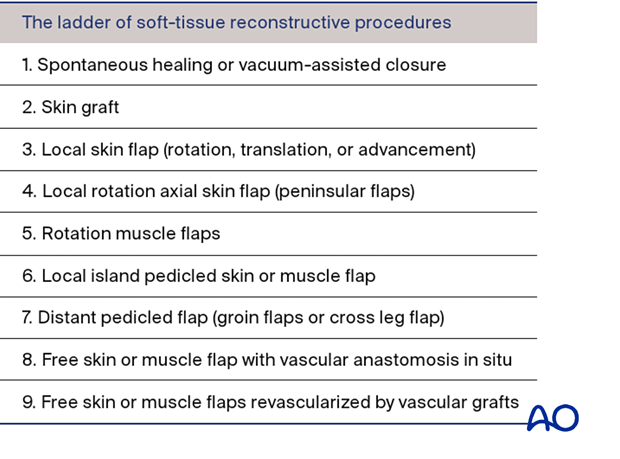
Eradication of infection and restoration of stability should be achieved before definitive wound closure. Wound closure can occur with local or free flaps, according to the “reconstructive ladder” model advocated by plastic surgeons. Definitive bone reconstruction can only occur with a healthy, closed wound.
Soft-tissue coverage
As a rule, soft-tissue coverage without complete debridement of underlying infected tissue is contraindicated.
A second rule is that wound closure under tension will usually fail. The wound edges necrose, the wound opens, and infection is unavoidable.

These rules apply to initial treatment as well as the management of infected fractures.
If direct suture closure is not possible, consider rotational muscle flaps, fascio-cutaneous flaps, or free vascularized flaps. Negative pressure dressings (vacuum-assisted closures) are very useful for some open wounds, or open soft-tissue defects.

Split-thickness skin grafting and secondary wound healing
If an open wound involves loss of skin and subcutaneous tissue, but has a base of healthy muscle, fascia, or tendon sheath, granulation tissue will form on the base and a split-thickness skin graft (STSG) can be applied, or the wound can be allowed to heal in from its sides (secondary intention).
A moist environment promotes granulation tissue formation. Muscles provide the best tissue for granulation.
Bare bone (without periosteum), exposed blood vessels, nerves, and tendons (without paratenon) are all harmed by desiccation and do not support granulation tissues and STSG. Alternative coverage techniques should be used. These tissues should not be left exposed and should be kept moist with appropriate dressings. Definitive coverage should be achieved as soon as possible.
The “reconstructive ladder” shown on the left presents, in increasing order of complexity, the options available for wound closure, and is helpful for treatment planning.
Bone graft options
Reconstruction of an infected fracture site can be done with autogenous bone graft, the Masquelet technique, or traction histogenesis (Ilizarov technique).
Autogenous cancellous bone graft from any standard site is of well-recognized value for promoting union and filling smaller defects. It revascularizes quickly, with minimal risk of sequestration. For treating tibial fractures, posterolateral, or central placement, adjacent to healthy muscle, may avoid the infected focus. For the humerus, femur, or forearm, the best position of the graft depends upon the defect, the soft-tissue envelope, and the fixation.
Bone defects that remain after resection of dead or infected bone are challenging to treat. The longer the defect, the more difficult the treatment. Circumferential defects of 1–6 cm in long bones usually heal if they are filled with autogenous bone graft and stabilized appropriately. Defects greater than this usually need traction histogenesis, free vascularized bone transfer, or bone grafting as per the Masquelet technique.
Traction histogenesis (Ilizarov)
Slow distraction of an osteotomy with stable (usually external) fixation creates new bone. Distraction is usually limited to 1 mm per day.
When matured, the new bone resembles the original bone in shape and strength. This technique, while slow, is particularly suitable for defects of more than 6 cm. This technique can be applied to a fresh osteotomy, distant from the site of the infected fracture, and also to a healing fracture site. Similar fixation can be used to compress a fracture, or osteotomy, to promote healing. Angular deformities can be corrected as well.
The techniques of traction histogenesis can be applied with any stable form of external, or even internal, fixation.
Diaphyseal bone can be transported over an intramedullary nail, which helps support the new (regenerating) bone. This allows earlier removal of the external fixation but introduces a foreign body with the risk of recurrent infection.
8. Amputation for osteomyelitis
Should repeated attempts to control infection, obtain bone healing, and restore function be unsuccessful, amputation may prove to be the most appropriate way of restoring function and allowing the patient to resume a more normal life.
The decision to amputate should be considered carefully by the people involved, including the patient and their family. One should compare the best predicted outcome from limb salvage with what is expected from amputation. The length of time required for treatment and complications that might occur during it are also important factors. Prosthetic function with a lower-extremity amputation, particularly below the knee, is much more successful than what can be achieved with upper-extremity amputations. The availability and cost of prosthetics must also be considered, and these vary in different health systems.
Different cultures, countries, and religions have different perspectives on amputation, and the individual patient’s preferences within his or her social setting must never be forgotten.
The surgeon’s job is to educate the patient about realistic expectations.
If, after due consideration, the patient decides that continued efforts at limb salvage are not desirable, the surgeon should recognize that amputation offers an opportunity for rehabilitation and restoration of function and plan the most functional level of amputation proximal to the diseased tissue. Cierny’s classification of host resistance to infection indicates that type A hosts are generally better candidates for limb salvage, while type B hosts, with impaired resistance to infection, may be better served by amputation. Type C hosts, for whom treatment is worse than their disease, are not surgical candidates unless their condition can be improved enough to tolerate surgery (either reconstruction, or amputation), or the infection flares out of control.
Consultation with a prosthetist may be helpful for the surgeon. The patient may wish to discuss amputation with another person who has undergone this procedure at a similar level. Once all of the infected tissue has been amputated, further infection treatment, beyond brief perioperative antibiotics, is no longer required. Just as with elective limb salvage surgery, efforts should be made preoperatively to help the patient attain the best possible nutritional and physiologic condition. In some instances, preliminary debridement or even a temporary amputation just above the infected wound may be needed to ensure success with the definitive amputation.

9. Case examples
Acute infection
In this Type A host with an infection developing only two weeks after fracture surgery, prompt operative treatment is necessary. This includes aggressive debridement, obtaining microbial cultures, and appropriate antibiotic treatment.
Fixation hardware should be checked for stability and left in place unless it is loose or prevents adequate debridement.
Antibiotics are begun as soon as cultures are obtained and are adjusted as necessary based on sensitivity results. Conventionally, they are continued for six weeks. Wound and fracture healing can be anticipated to proceed uneventfully.

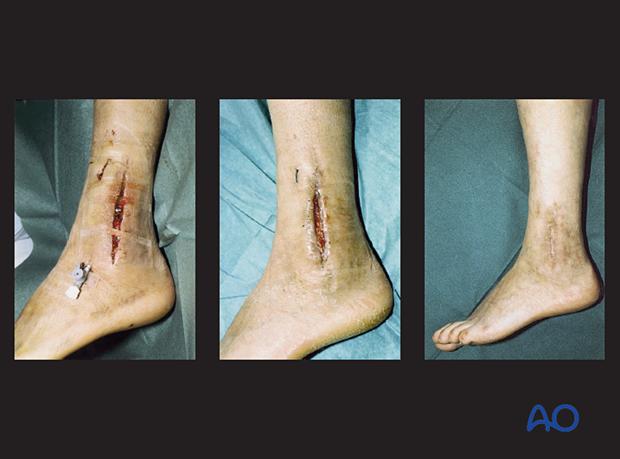
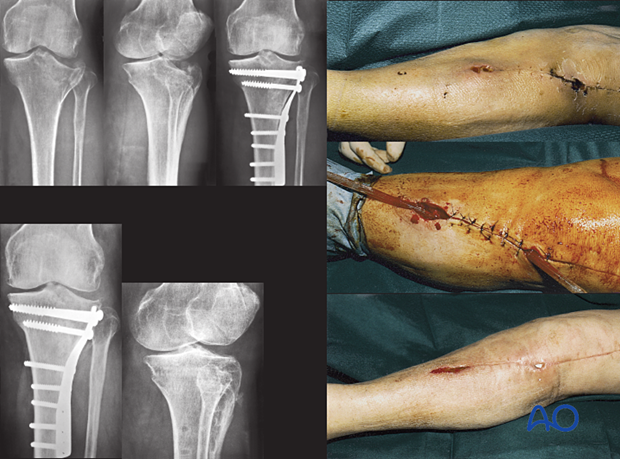
Delayed presentation – 2–6 weeks (hidden infection)
This Type A host had open reduction and internal fixation (ORIF) of her lateral plateau fracture. Healing was slow, and she returned with an open wound 24 days later. She immediately had aggressive debridement and antibiotics for two months. The wound and fracture then healed uneventfully.
The hardware was retained, but may have needed removal, or complete revision, if instability had been evident, or the infection failed to resolve.
Once the fracture was securely healed, the hardware was removed.
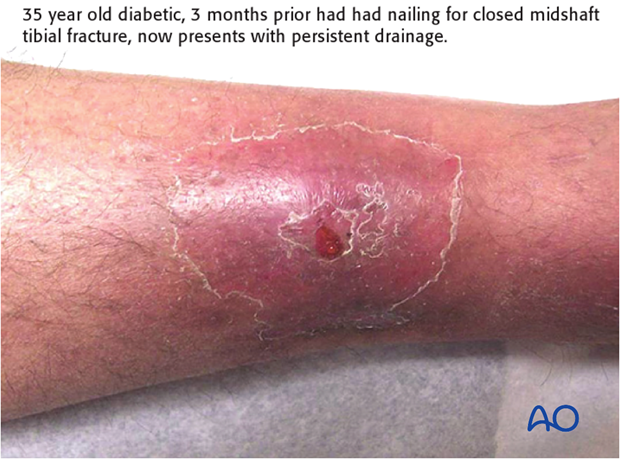
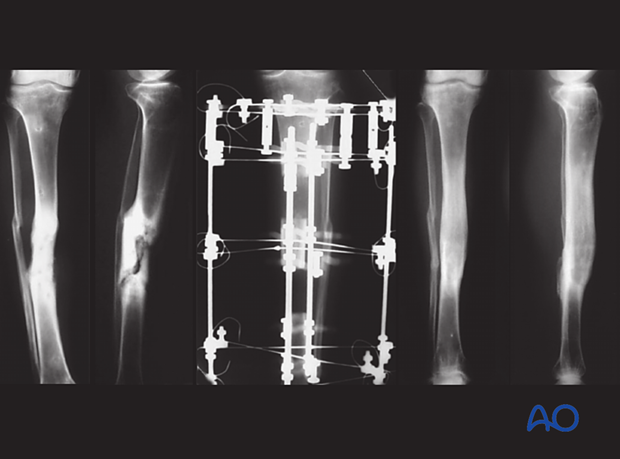
Chronic infection (>10 weeks)
This Type B host with type 2 diabetes presents with persistent drainage three months after having intramedullary nailing for a closed tibial shaft fracture. He needs to have careful diabetic control as the infection will make his diabetes less responsive. Furthermore, diabetes mellitus, especially if poorly controlled, reduces host resistance to infection.
Once the medical condition is stabilized, surgical debridement can be performed. This involves debriding the sinus tract, removing all the hardware, and irrigating the medullary canal after re-reaming to a larger size. After obtaining cultures, appropriate antibiotics are started and continued for at least six weeks. If the fracture is healed, no further interventions are required, unless infection recurs.
If the fracture has not healed, refixation will be necessary with either an external fixator or new IM nail.

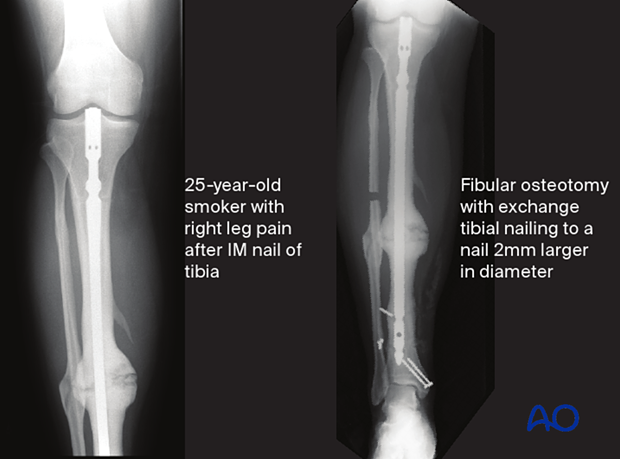
Septic hypertrophic nonunion
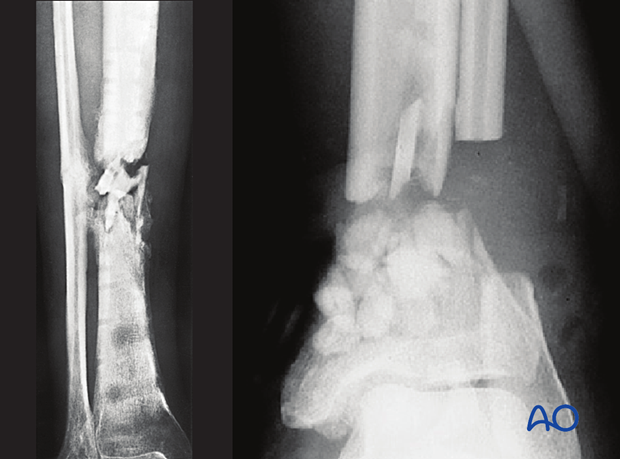
The image on the left shows a case of a young man, a Type B host, who required smoking cessation education when he had his original trauma. He developed a presumed aseptic hypertrophic nonunion of a diaphyseal tibia fracture that underwent IM nailing. The nail was removed, and a short segment of fibula was excised. The tibia was re-nailed after reaming to a larger diameter nail. The reamings were sent for culture and came back positive. Appropriate antibiotics were given for 6 weeks after surgery. The tibia healed without complication.
Septic atrophic nonunion
This patient had an acute fracture, necessitating internal fixation. However, a late infection occurred. X-rays and subsequent debridement revealed an ununited fracture with minimal callus formation. Intercalary necrotic bone was debrided. The fracture was stabilized with an external fixator.
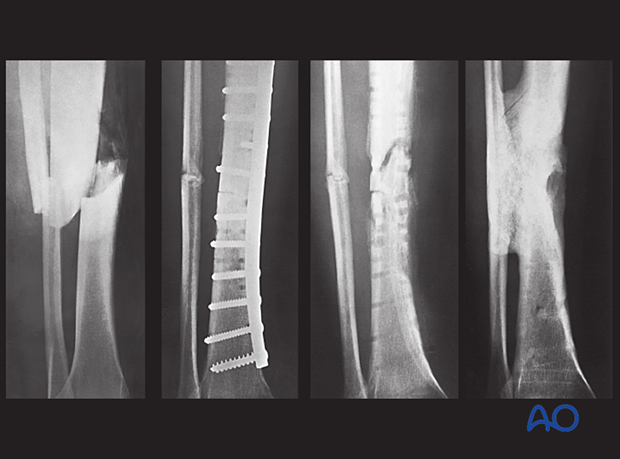
Appropriate antibiotics were administered. Bone graft was then placed in the fracture defect and between the tibia and fibula above and below the fracture. This combination of treatments improved both the biology and stability of the atrophic nonunion. Delayed healing occurred with a satisfactory outcome.
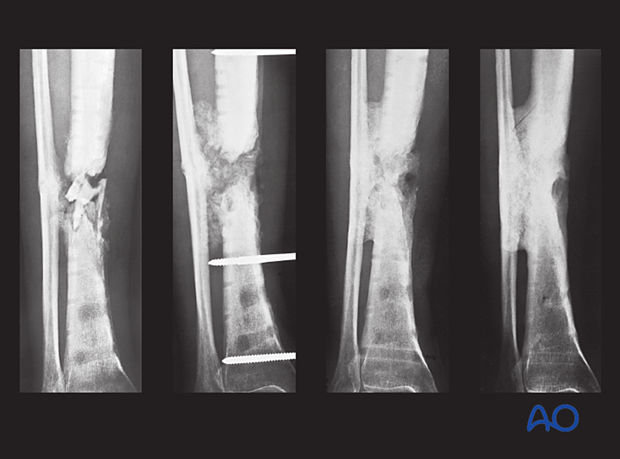
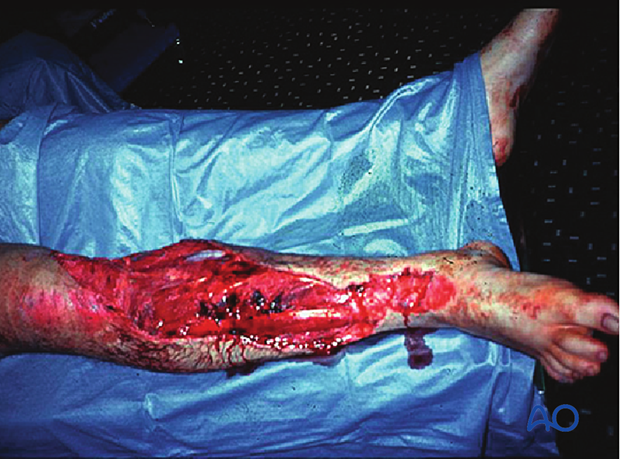
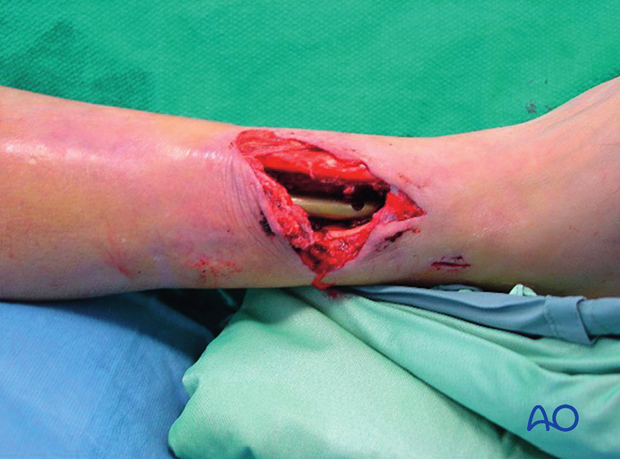
Infection leading to amputation
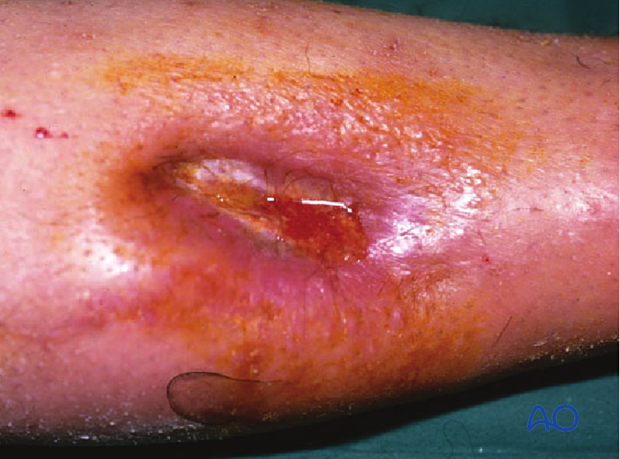
This 40-year-old motorcyclist had had acute fixation of an open pilon fracture. The picture on the left shows the wound ten days after open reduction and internal fixation. Skin edges were necrotic and the patient was acutely septic.
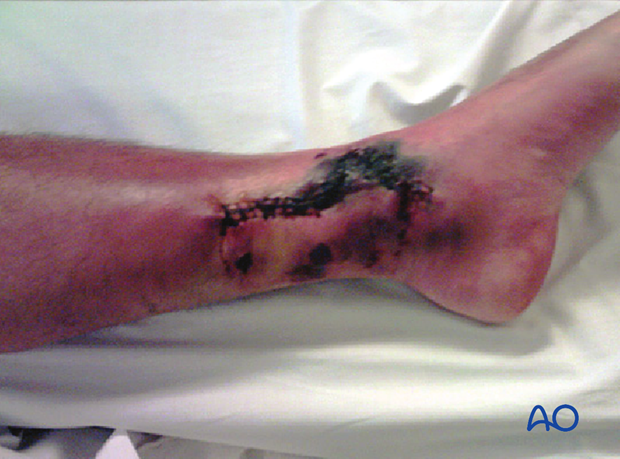
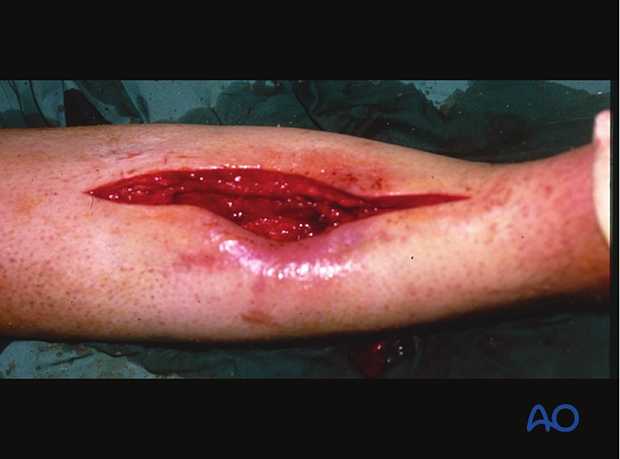
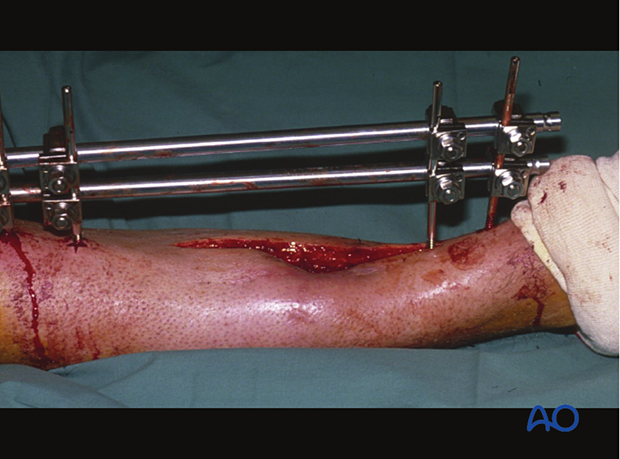
This intraoperative image shows loss of soft tissue and bone. Extensive debridement was undertaken to control the infection. Systemic antibiotics were administered. This patient was found to have clostridial gas gangrene. To save his life, the limb was amputated.