Posterior decompression and stabilization C7-T1
1. Introduction
Preoperative planning
Based on CT and MRI imaging, a plan should be prepared to determine:
- The size and optimal location of implants used
- Whether spinal cord decompression is necessary and if so, the amount of tissue to remove in order to achieve sufficient spinal cord decompression
Every case will be unique, and we will here illustrate just one example.
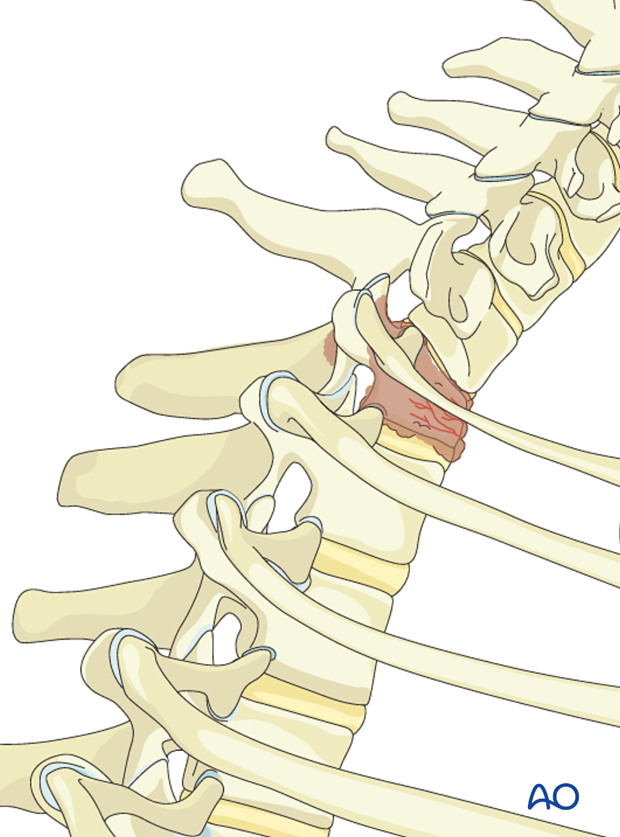
The cervico-thoracic junction
The cervico-thoracic junction has a specific biomechanical behavior. This is due to the sudden change from the extremely flexible cervical spine, to the more rigid thoracic spine.
The thoracic spine has a shorter elastic zone due to:
- the rigid rib cage.
- the coronal orientation of the facet joints.
- the overlapping of the spinous processes.
Biomechanically the cervico thoracic junction is vulnerable to kyphosis and shear forces. Usually a long construct (2-3 levels above and 2-3 below the tumor) is necessary to achieve adequate stability. That is why the anterior alone constructs at this level are not recommended because of high-failure risks.
The anterior approaches might be difficult because of complex anatomy involving numerous essential anatomical structures at this level.
Use of dexamethasone
Administration of dexamethasone (10 mg bolus, 4 mg every 6 hours) is recommended as soon as ESCC causing neurologic deficit is diagnosed. Dexamethasone should be stopped or tapered as soon as spinal cord decompression is completed.
Embolization
Embolization procedures are recommended to reduce operative blood loss in hyper vascular tumors, especially for larger resections.
Neurological evaluation
The preoperative neurological assessment must be carried out as described in the Neurological Evaluation.
2. Patient positioning and surgical approach
For this procedure the patient is placed in the prone position and the posterior access to the cervicothoracic junction is used.
3. Screw insertion
Screws are inserted at least two or three segments above and below the tumor.
In tumor patients achieving optimal screw purchase is even more important than in trauma patients to minimize risk of pullouts and reduce the number of levels involved.
Optimal pedicle screw purchase will, in order of importance, be achieved by:
- Selecting the largest possible screw diameter
- Selecting the longest possible screw
- Positioning of the screw under the cranial endplate
- Cement augmentation of the screw.
Cervical screws
In the lower cervical levels, the following screws can be used:
Due to safety, lateral mass screws are preferred from C3 to C6. If the lateral mass is compromised an additional level can be included in the fixation or pedicle screws may also be considered.
A lateral mass screw can also be used at the level of the tumor if the lateral mass is intact.
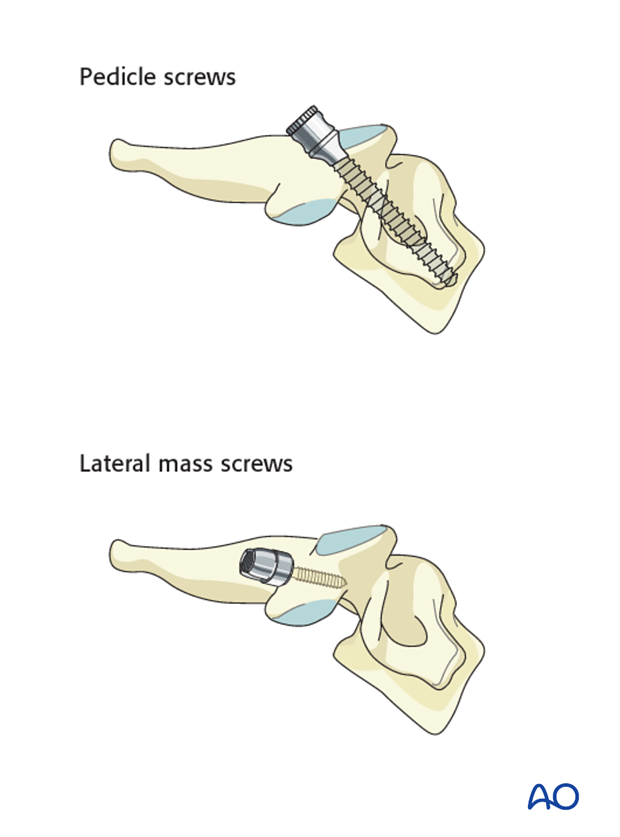
Thoracic screws
In the upper thoracic spine, the following screws can be used:
If the pedicles are too small (extremely rare for the T1 level), an outside-inside technique or laminar screws can be used as an alternative at the level of T1-T2.
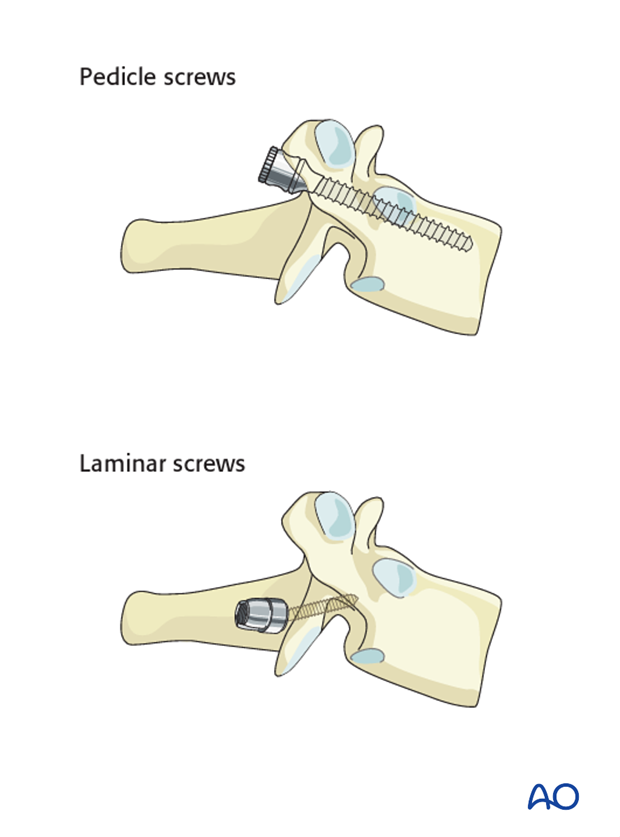
4. Rod insertion
Rod selection
Double diameter tapered rods can be used if available. In this case no rod connectors are needed.
This is the preferred option as it is easier to install and reduces operating time compared to other constructs. Alternatively, stabilization of the cervicothoracic junction can be performed with a cervical system provided 4.0mm rods and pedicle screws are available.
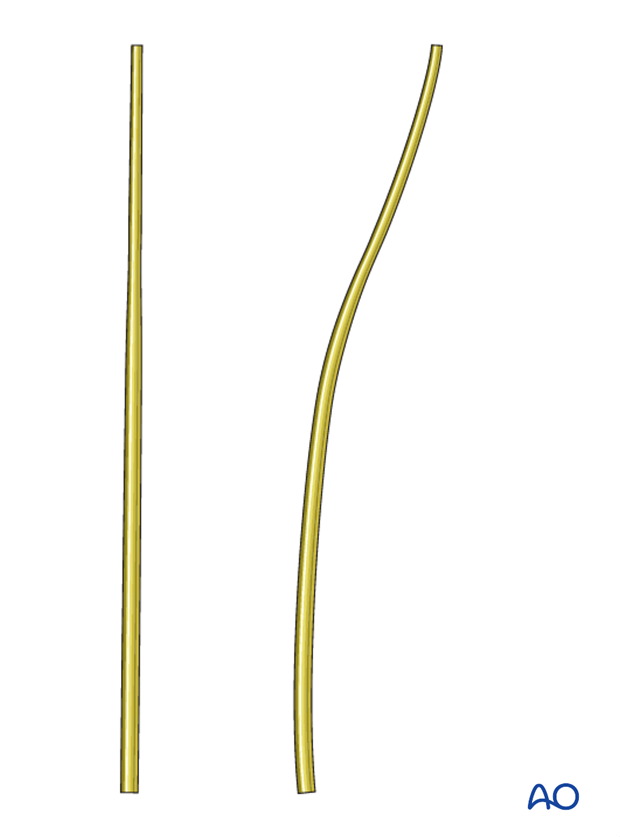
Rod contouring
Rod contouring should mainly follow the curvature of the spine. Reducing preexisting deformities is typically not necessary and may lead to screw pull-out.
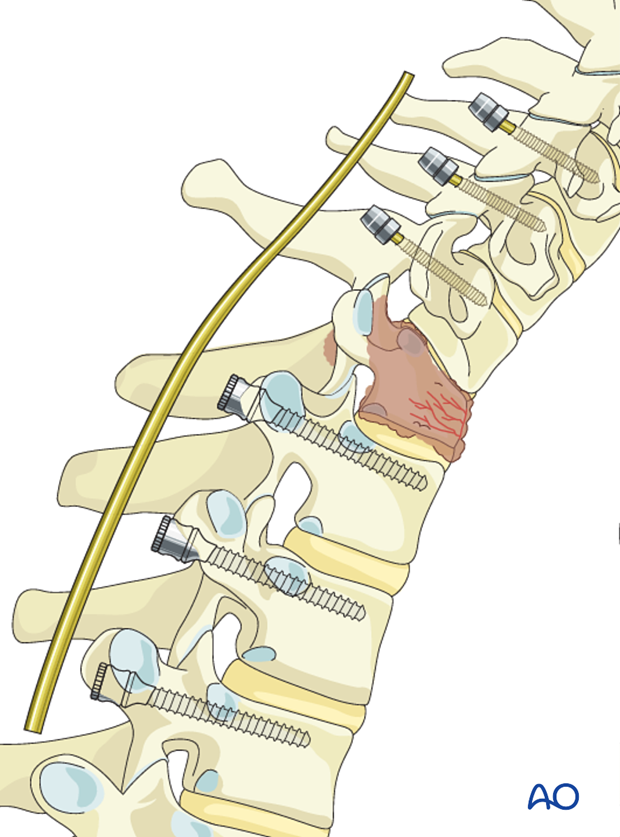
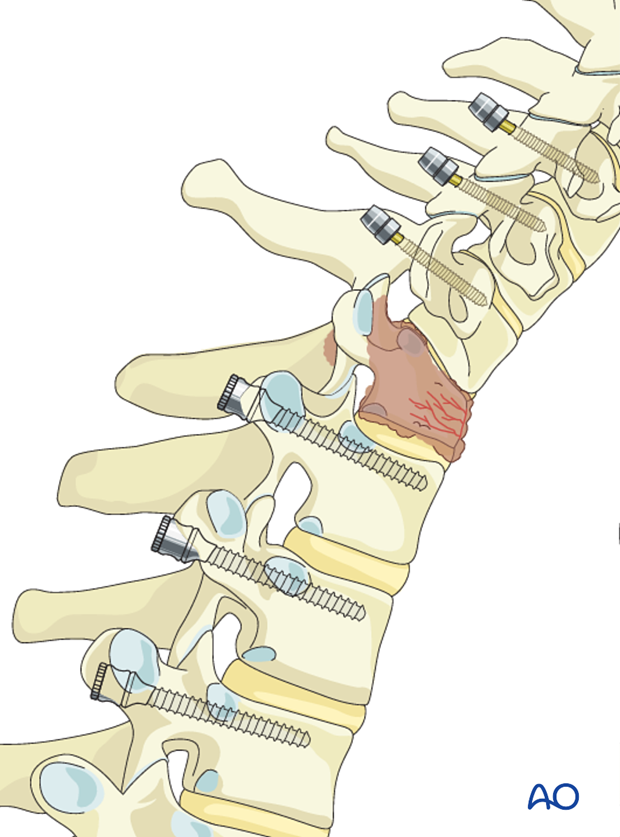
The rod is inserted into the screw heads and the screw heads are tightened with the inner nuts.
If posterior decompression is performed, the insertion of only one rod can facilitate access to the spinal canal. To illustrate this procedure, we will show the decompression with only the first rod in place.
5. Decompression
Timing of decompression
A patient who is experiencing neurologic deficit from solid tumor ESCC resulting in loss in ability to ambulate, in the absence of medical and oncological contraindications, requires urgent surgical decompression.
Expeditious diagnosis and prompt surgery are recommended to improve the probability of neurological recovery.
A laminectomy is performed in the area between the pedicles of the vertebra.
Based on preoperative planning, the amount of lamina to be removed to achieve enough decompression can be determined beforehand.
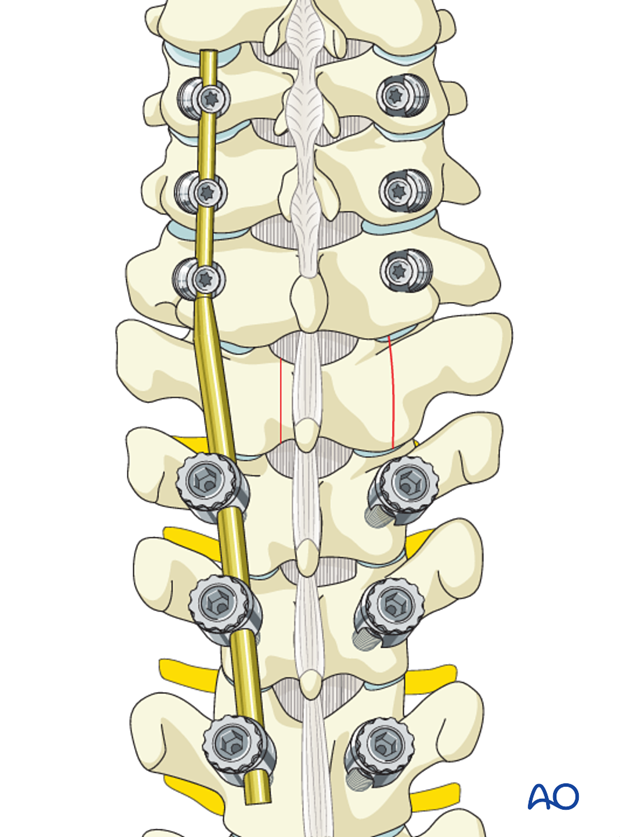
Remove the spinous process and the lamina in one piece.
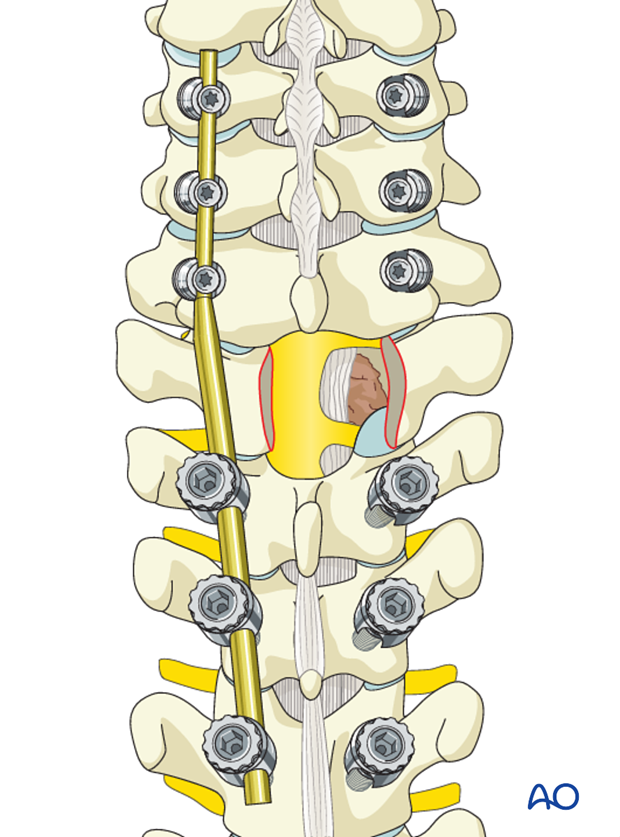
The adjacent spinous processes and the intervening ligamentum flavum are excised.
Portions of the superior and inferior lamina and the medial portion of the facet joint are removed.
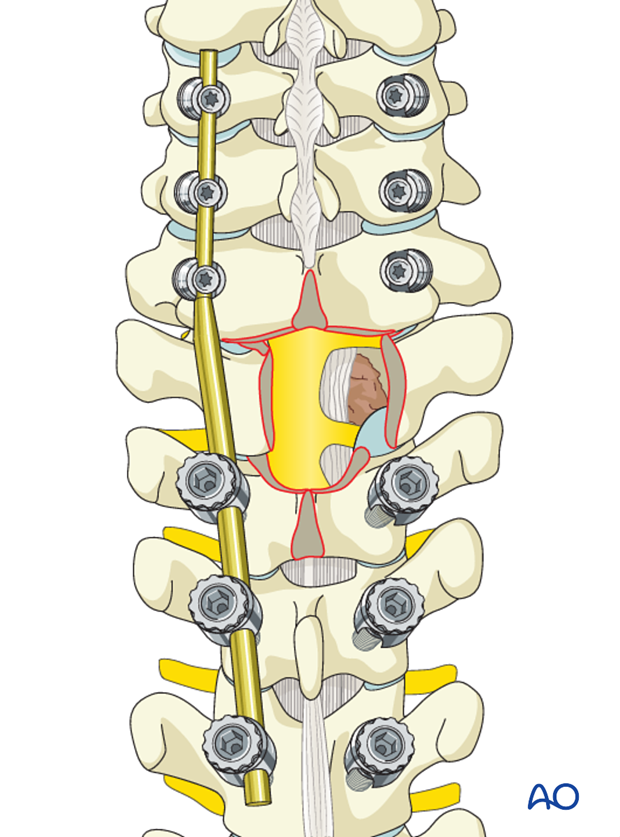
The posterior longitudinal ligament (PLL) should be identified and separated from the dura with a dura dissector.
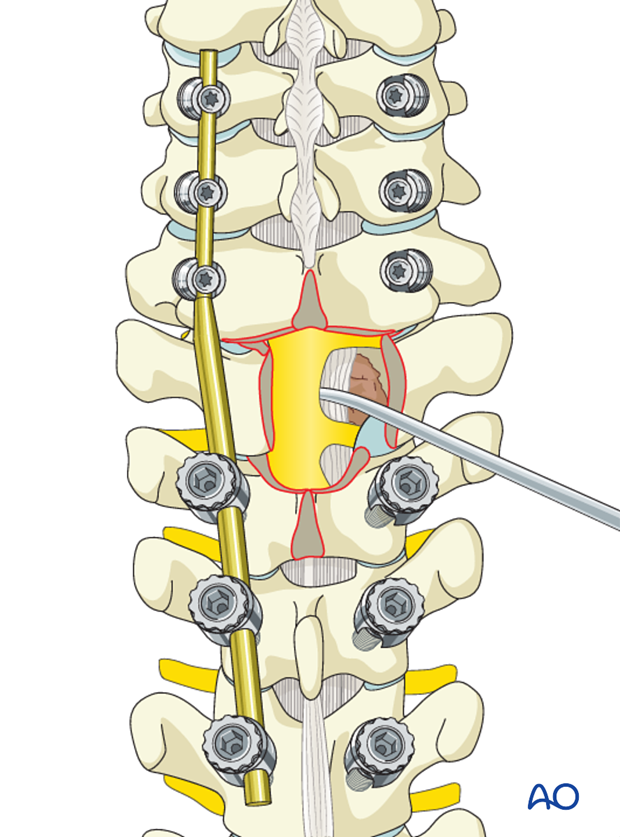
The PLL should be cut in order to expose the vertebral body and the epidural tumor. This circumferential spinal cord decompression technique coupled with postoperative SBRT is known as separation surgery.
The spinal cord, dura, and tumor should be manipulated as little as possible.
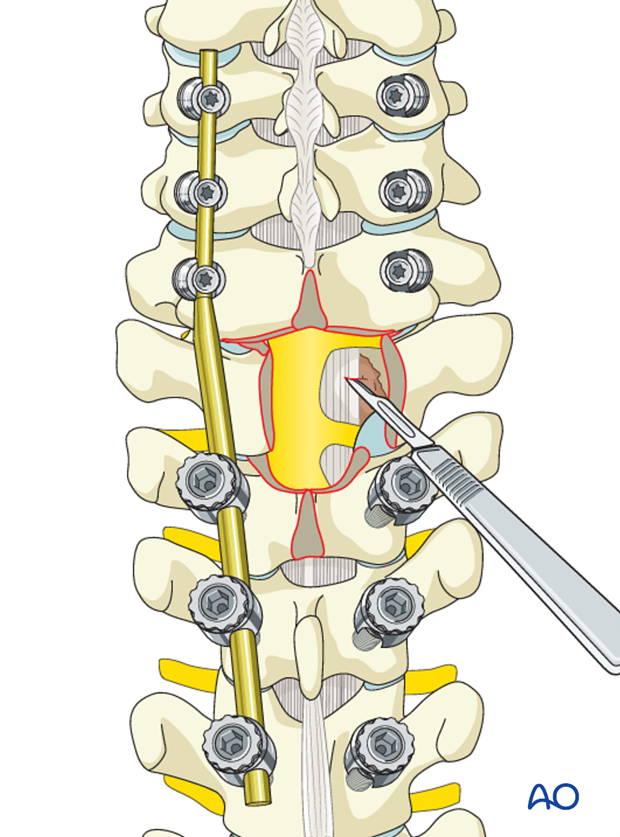
Reverse-angle curettes and pituitary rongeurs can be inserted through this opening, and any tumor and bone fragments compressing the anterior neural elements can be removed or impacted into the vertebral body.
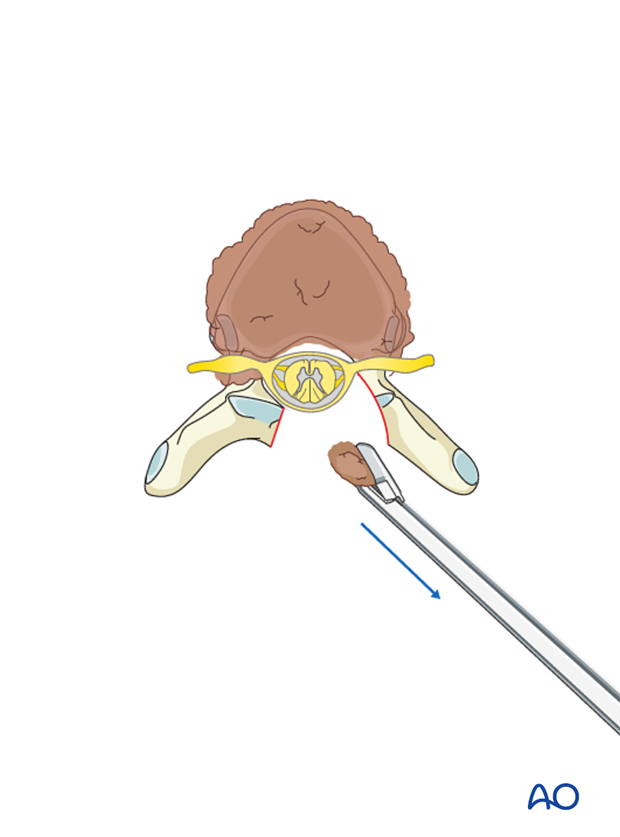
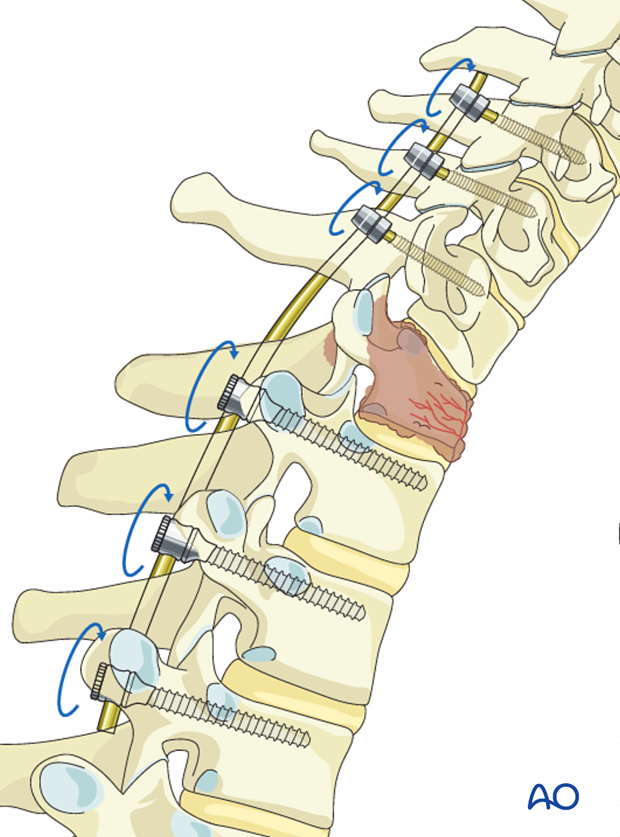
If further decompression is needed, the same technique may be applied on the other side.
If the decompression is adequate on both sides of the canal, the second rod may be fixed and tightened.
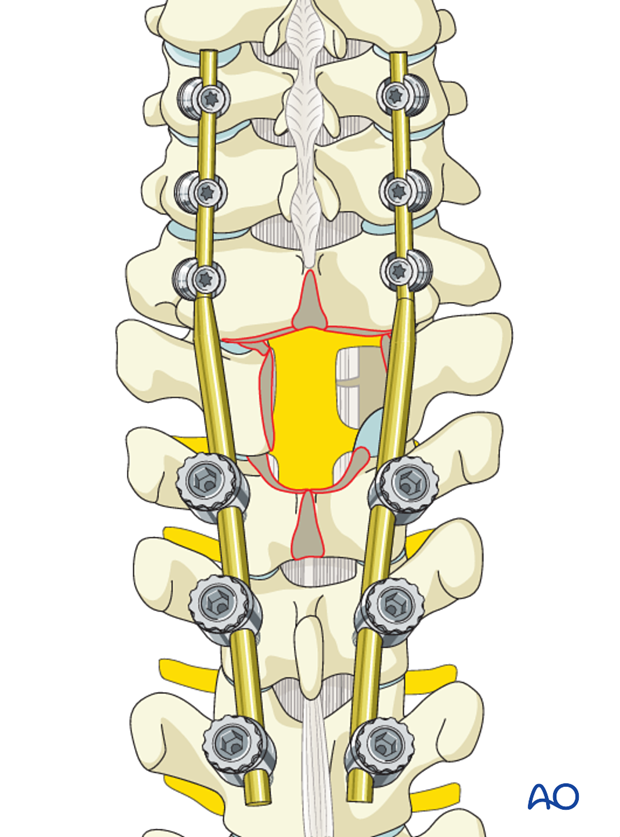
6. Fusion
Decision
Live expectancy and performance status should be used to determine whether bone grafting is indicated.
For patients with good prognosis and a long life-expectancy, posterior fusion may optionally be performed using allograft and/or local autograft.
If the surgeon plans for a fusion, the facet capsule is excised and the joint cartilage surfaces are denuded/curetted.
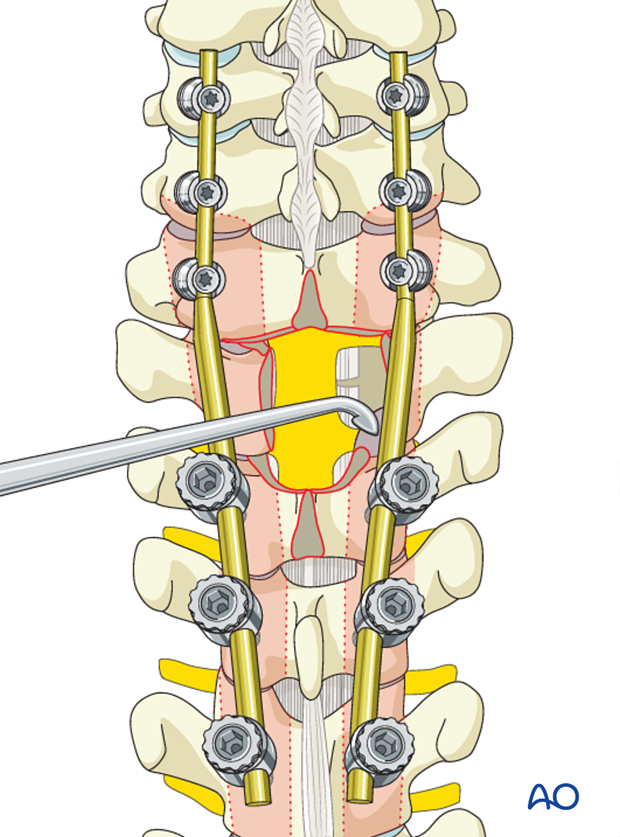
Pieces of bone graft (autograft, allograft) are inserted into the decorticated facet joint for fusion.
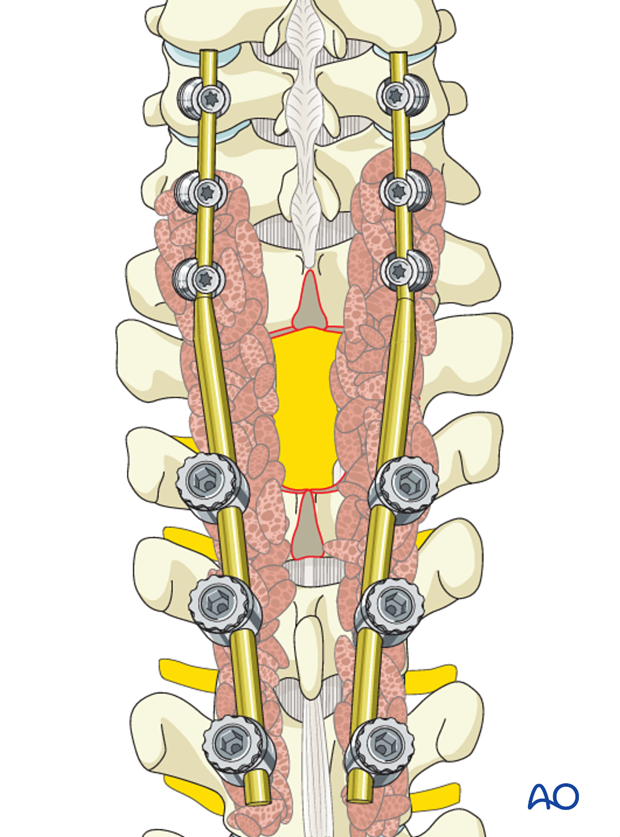
7. Postoperative imaging
Postoperative AP views should be obtained. As lateral views are not available in this area, a CT scan should be obtained as soon as possible after surgery to confirm the placement of the instrumentation.

8. Aftercare
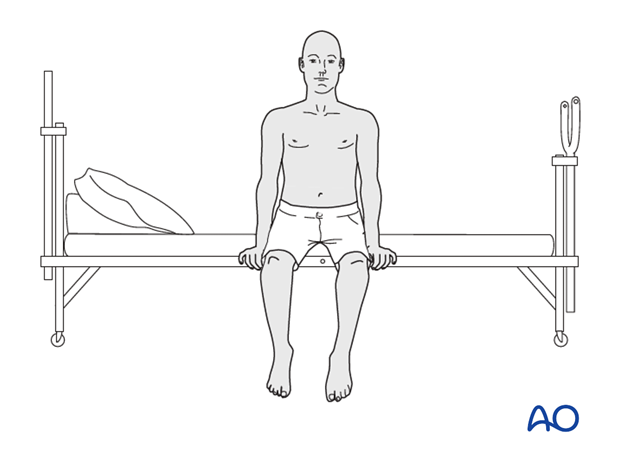
Patients are made to sit up in the bed on the first day after surgery. Bracing is optional but preferably omitted for patient comfort. Patients with intact neurological status are made to stand and walk on the first day after surgery.
Patients can be discharged when medically stable or sent to a rehabilitation center if further care is necessary.
During admission adequate caloric intake of a high-quality diet should be monitored.
Patients are generally followed with periodical x-rays and (optionally) MR imaging at 6 weeks, 3 months, 6 months, and 1 year to monitor for tumor recurrence and hardware failure.
Postoperative radiation is required to avoid tumor recurrence. SBRT is usually initiated within two weeks following surgery. Conventional radiotherapy is usually initiated 2-4 weeks after surgery to reduce the risk of wound healing disturbances.
The radiation modality is selected based on tumor histology and history of prior radiation.













