Principles of management of open fractures
1. Classification of open fractures
Introduction
In 1895, Stanley Boyd said “The most important divisions of fractures - simple, compound and complicated - are based upon the condition of the soft parts.”
There is no doubt that the status of the soft-tissue wounding in open fractures is a crucial determinant of the outcome.
Most research shows that the infection rate increases with the severity of the soft-tissue injury. Less important is the bony injury, provided that the bone that has been injured has a blood supply.

Common classifications
The classification of such soft-tissue wounding is according to two systems, namely that of Gustilo, Mendoza & Williams (See: Gustilo RB, Mendoza RM, Williams DN (1984) Problems in the management of type III (severe) open fractures. A new classification of type III open fractures. J.Trauma Aug;24(8):742-6); and also that of the AO.
An additional influence is the ability of the host to combat infection, based on both systemic and local factors. For details see Cierny classification.

Wound-severity classification
Gustilo and Anderson. (JBJS 1976)
This work largely addressed lower leg injuries, but has some value in other anatomical sites.
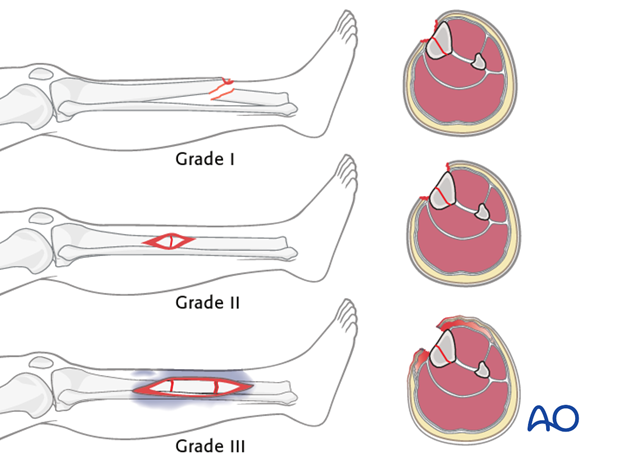
The Gustilo – Anderson classification divides soft-tissue wounding of open fractures into three grades – I, II & III.
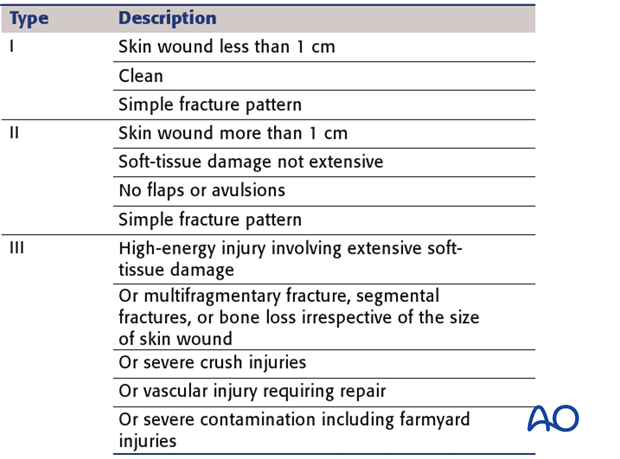
This illustration summarizes the three basic grades – I, II & III
Gustilo, Mendoza and Williams. (J.Trauma 1984)
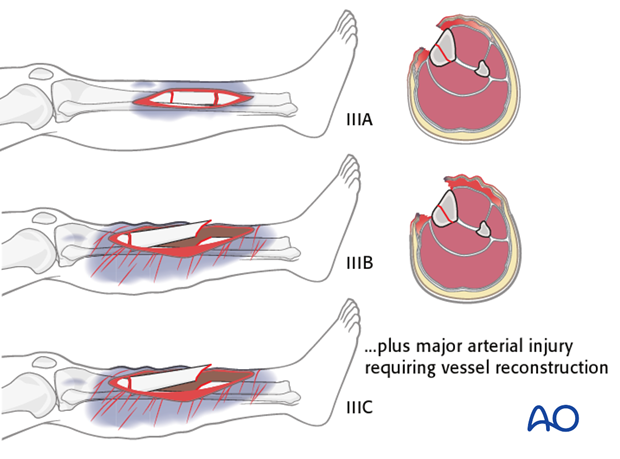
The III grade was later further subdivided into types IIIA, IIIB & IIIC.
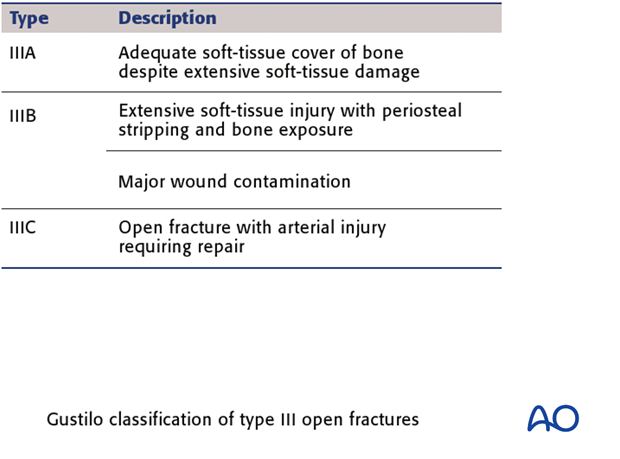
These examples illustrate the three types IIIA, IIIB & IIIC.
AO classification (adapted from Tscherne)
The AO classification of fracture wound severity provides a grading system for injuries of each of the skin (I), muscles and tendons (MT), and neurovascular (NV), each of which is divided into five degrees of severity.
It is designed to provide a unique, unequivocal definition of any injury and, thereby, allows accurate comparison of cases.
A full understanding of the severity of an open fracture requires consideration of each of these soft-tissue elements.
This very detailed classification is designed to be used in conjunction with the AO/OTA Fracture and Dislocation Classification.
The detailed categorisation of open fractures by the AO system is most reliably done in the operating room at the completion of primary wound care and surgical excision.
When used in a large database this multifaceted, alphanumeric classification permits very precise comparison of injury types and is most useful as a research tool. However, its complexity renders it largely impractical for everyday use in clinical practice.

Comparative example
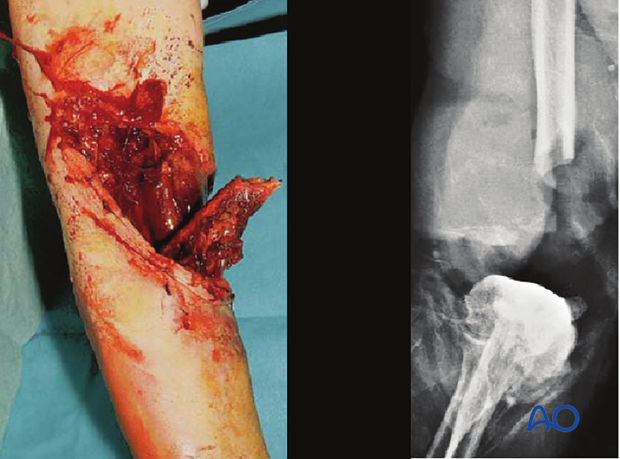
The Gustilo-Mendoza-Williams open-fracture classification separately identifies, as type IIIC, those grade III open fractures with arterial injuries that require vascular repair to restore limb viability. Gustilo et al. demonstrated a 50% risk of osteomyelitis after such injuries, with amputation (early or late) a frequent outcome ( Gustilo et al. (1990) The management of open fractures. J Bone Joint Surg 72(2):299-304).
This illustration shows a severe open injury of the shaft of the lower humerus, after a high-energy motor vehicle collision. There was disruption of the brachial artery and vein and neurapraxia of the median, radial, and ulnar nerves. It would be classified as a Gustilo IIIC injury, whereas on the more comprehensive AO/OTA Fracture and Dislocation Classification it would be a 12-C3, IO4-MT4-NV4.
2. Principles of surgical care for open fractures
Introduction
Open fractures need
- prompt diagnosis
- appropriate intravenous antibiotics
- meticulous injury zone excision (débridement) *
- fracture stabilization
- second look
- early soft-tissue cover after soft-tissue recovery
* Such surgery is frequently referred to as débridement. This term is open to interpretation and denotes different procedures in different surgical contexts.
Débridement, as used in this discussion, means the surgical exposure of the whole pathological injury zone and the removal of all necrotic, contaminated, and/or damaged tissue, whether bony or soft-tissue.
Intravenous antibiotics for open fractures
Antibiotics for open fractures are an adjunct to meticulous wound débridement (see Pearl below).
Bacterial contamination is always present with open fractures. Bacterial count and infection rate can be significantly reduced by prompt administration of intravenous antibiotics, in combination with surgical débridement.
Most infecting bacteria, except in very dirty wounds, are typical skin flora. A first generation cephalosporin (e.g., cefazolin 1-2 grams/8 hours) is often used, except for patients with penicillin allergy.
For more severe open-fracture wounds, add an aminoglycoside (eg., gentamycin 80 mg/8-12 hours).
If “agricultural” contamination is present, high-dose intravenous penicillin is usually added (e.g., 5 million-10 million units/24 hours) and consider metronidazole.
They should be started as soon as the open fracture is diagnosed, but continued for only 2-3 days.
Pearl: “It is irrational to hope that a short course of antibiotic prophylaxis can cure fundamental surgical errors” (Geroulanos & Hell (1989) Antimicrobial Prophylaxis in Surgery).
Intraoperative wound contamination
A key principle of safe surgical treatment is to minimize the number of bacteria that might enter the surgical wound. Appropriate preoperative skin decontamination, with washing using antibacterial agents, is a mainstay of this.
Similarly, the use of sterile drapes, instruments and implants, and the maintenance of strict aseptic discipline throughout the procedure are also important.
In the absence of optimal sterility, or with overwhelming surgical load, only the most limited emergency surgery should be carried out (e.g., emergency wound excision).
3. Débridement
Débridement of the injury zone in open fractures
The injury zone excision must be complete, meticulous and radical.
Early wound débridement is the most important component of the care of any open fracture.
The surgical site should be thoroughly irrigated (several liters of fluid – optimally, a balanced salt solution, such as Ringer-lactate - to reduce the bacterial population) (see next step). The epithet “dilution is the solution to pollution” has certain merit in this context.
In cases with significant amounts of contaminated, dead, or possibly ischaemic, tissue, additional wound excision 48 hours later (second look) is often necessary – if in doubt, look again.
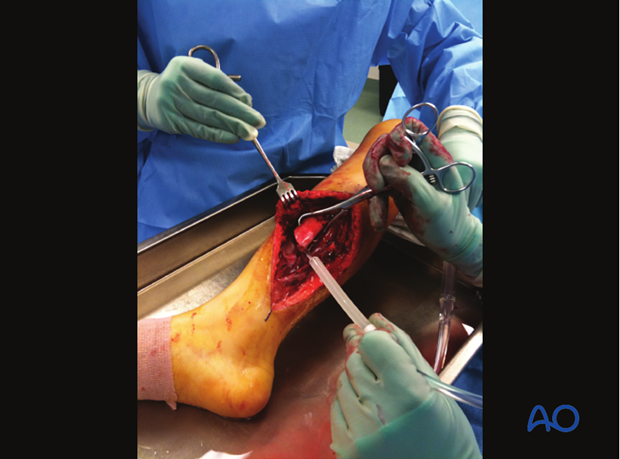
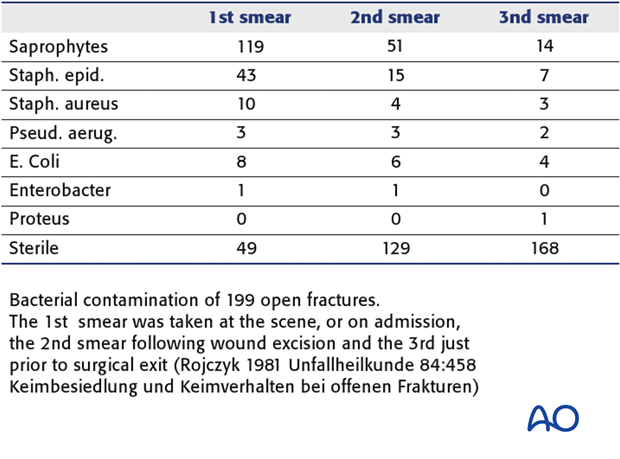
Tscherne & Gotzen’s book “Fractures with Soft Tissue Injuries” (1984. Springer-Verlag. pp.19-20) refers to the work of Rojcyk (1981) as follows: ”During the operation the wound is irrigated repeatedly with Betadine or Ringer’s solution. Following the débridement, all surgical instruments and attire are changed, and the wound is redraped as for a new operation. The benefits of this routine are demonstrated in a continuous series of 199 open fractures (see table). The number of positive microbiological smears decreases markedly from the initial contamination by the trauma to the end of the operation.”
Deciding which tissue to remove and which to retain is the essential challenge of wound débridement. This is best learned in the operating room from senior surgeons and by supervised practice. Typical errors are failure to remove enough compromised tissue, or to do so in a way that causes additional injury to retained healthy tissue.
Take an organized approach that proceeds in orderly steps through each tissue layer. First, enlarge the traumatic wound for adequate exposure of the whole injury zone. Only minimal non-viable wound margins need to be excised. Explore the depths of the injury zone, and examine it thoroughly. Protect and preserve major blood vessels and nerves, tendon sheaths, healthy periosteum and soft tissue attached to bone.
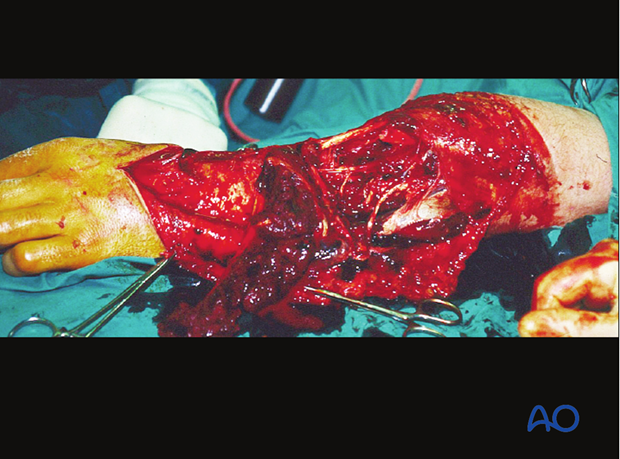
Next, all dead, or questionably viable, tissue is excised systematically from each tissue layer:
- subcutaneous tissues
- deep fascia
- muscle
- bone
At each level, leave only obviously viable tissue.
Any bony fragments devoid of soft-tissue attachment should be removed. Contaminated, or non-viable, bone surfaces will also need excision with hand instruments, such as chisels and rongeurs.
Copious irrigation with a balanced salt solution (such as Ringer-lactate) helps to remove bacteria, bits of dead tissue and blood clot, and improves the surgeon’s ability to examine the wound.
The use of pulsed pressure-lavage systems risks driving contamination into the hidden depths of the wound, and is of questionable value.
See also:
- Bhandari, M. et al. (1999) High and Low Pressure Pulsatile Lavage of Contaminated Tibial Fractures: An In Vitro Study of Bacterial Adherence and Bone Damage. J Orthop Trauma 13: 526-533.
- Hassinger, S.M. et al. (2005) High-Pressure Pulsatile Lavage Propagates Bacteria into Soft Tissue Clin Ortho Rel Res 439; 27-31.
- Crowley, D. J. et al. (1989) Irrigation of the wounds in open fractures J Bone and Joint SurgB - 89, 580-585.
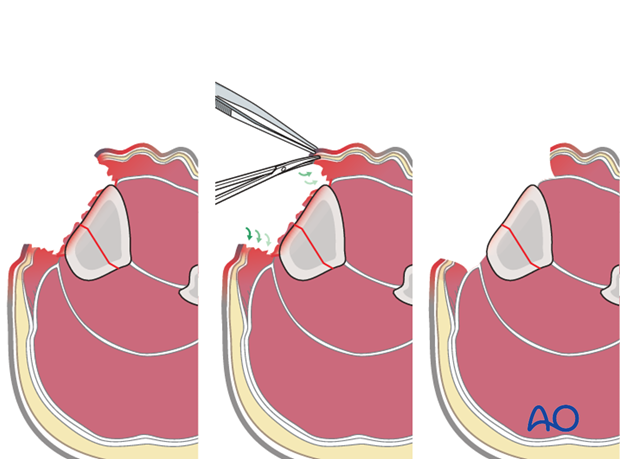
Fractures with open joint injuries
When an open fracture communicates with a joint cavity, special surgical tactics are required.
As always, all devitalized tissue must be removed. Joint surfaces should not be allowed to become dry.
If possible, the open joint itself should be closed primarily. If this is not possible, the joint must be kept clean and moist (moisture-retaining dressing). Negative pressure wound therapy (NPWT) or VACs cannot be used over top of a joint.
Early definitive closure should be planned.
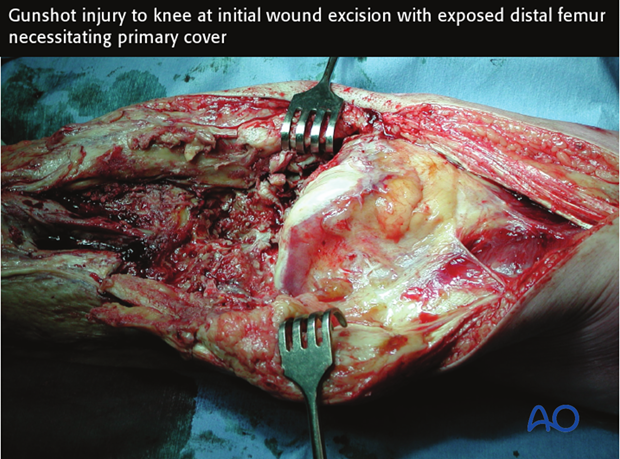
4. Fixation of open fractures
Open fractures need
- surgical stabilization, usually external
- delayed definitive ORIF.
Bony stability in open fractures helps associated soft-tissue wounds to recover, by providing the best possible setting for soft-tissue healing and resistance to infection.
See:
- Worlock P, Slack R, Harvey L, Mawhinney R. (1994) The prevention of infection in open fractures: an experimental study of the effect of fracture stability. Injury: 25(1):31-8.
- W. W. Rittmann, S.M. Perren, M. Allgöwer and F.H. Kayser (1975) Cortical Bone Healing after Internal Fixation and Infection: Biomechanics and Biology. Springer Verlag, Berlin.
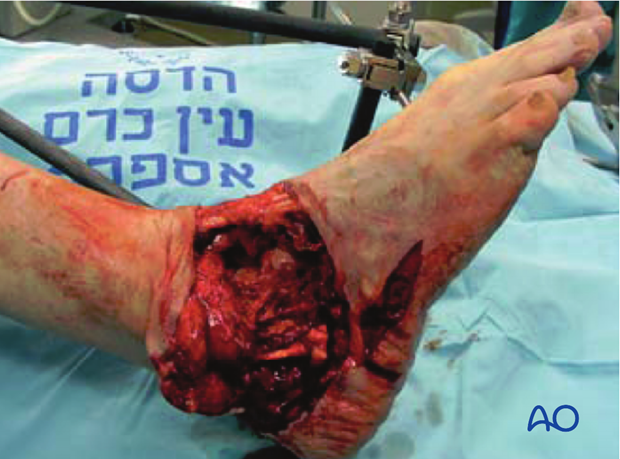
Surgical fixation, external, or internal, is the best way to stabilize an open fracture. This is done only after thorough injury zone débridement.
For lower-grade, open fractures, use fixation that would be appropriate for similar closed injuries. For more severe open fractures, or wounds that need repeated excisions, external fixation is usually preferable.
Intramedullary nailing (IMN) is occasionally chosen as fixation for low-grade femoral, or tibial, diaphyseal open fractures.
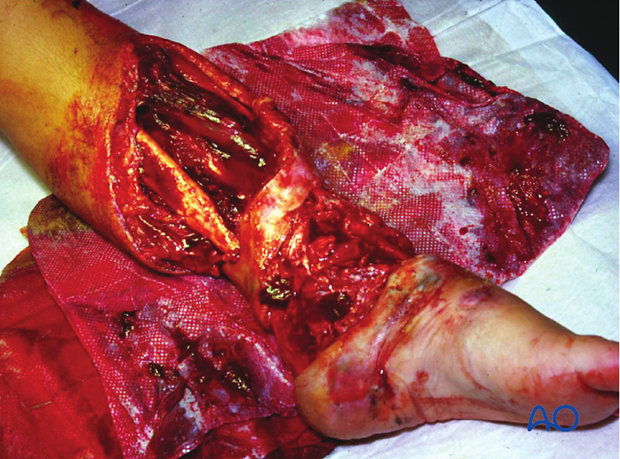
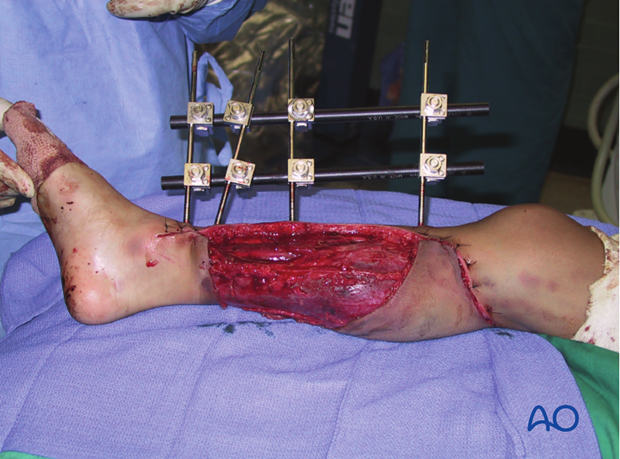
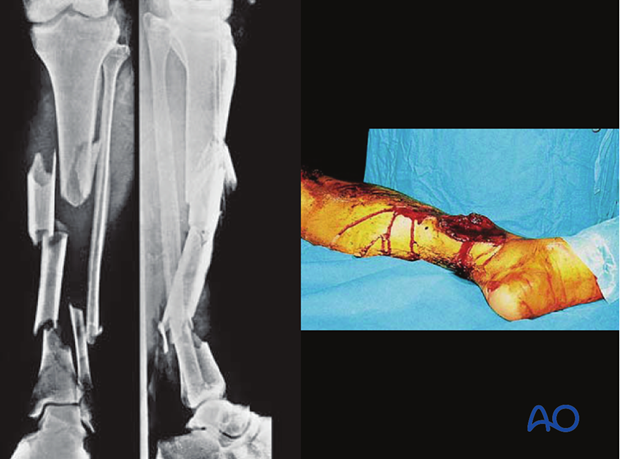
These illustrations show a severe open segmental tibial fracture, in which, short of primary amputation, IMN, using an unreamed solid nail, was the only realistic alternative, despite the risks.
If IMN must be delayed (significant wound contamination, etc.), temporary external fixation can be used for preliminary stabilization.
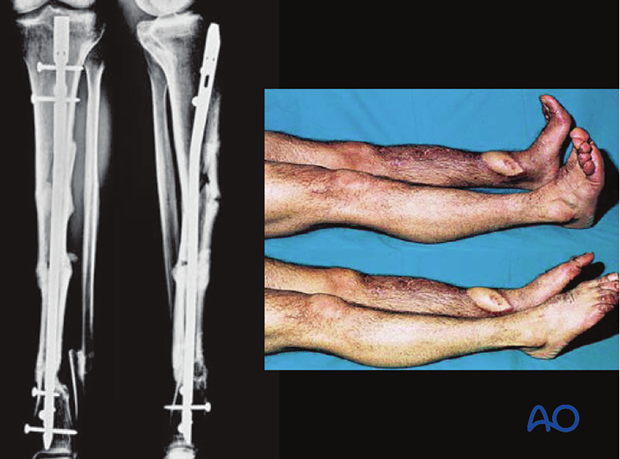
Images showing healed severe tibial fracture with unreamed tibial nail in place.
5. Soft-tissue care
Open wound care
- Avoid contamination
- Avoid desiccation
- Consider special dressings
- Cover promptly
Any open wound needs to be protected from secondary contamination. A sealed dressing (e.g., antibiotic bead pouch, or vacuum-assisted wound closure (VAC)) can be used. VACs help to reduce the size of an open wound and promote the formation of granulation tissue. It may permit early split-thickness skin graft closure.
Closure with local, or free flaps is appropriate for larger and more complicated wounds and for open joints, as soon as staged wound excision is complete.
It is important to close a complex wound, especially involving a joint, as soon as the wound appears healthy (preferably at 5-7 days), rather than to leave it open and risk hospital infection.
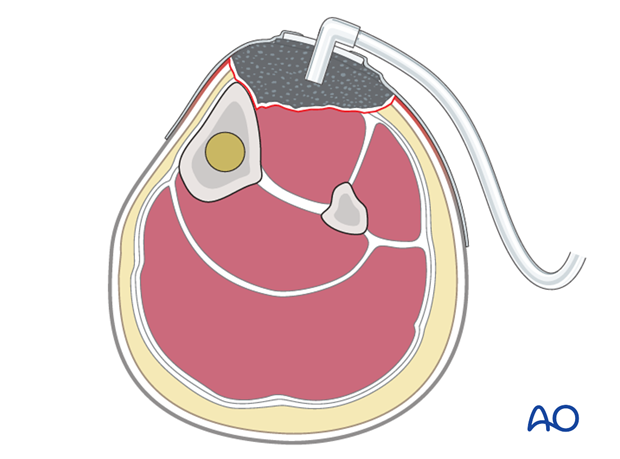
Soft-tissue coverage in open fractures
- Only ever after wound excisions are complete
- Delayed closure of the traumatic wound is safer in all open fractures.
After wound débridement has been satisfactorily completed, in either one or more procedures, consideration must be given to the best means of wound coverage. Excessive skin tension will prevent wound healing. Furthermore, a contaminated wound is virtually certain to become infected with primary closure.
Temporary open wound management with delayed primary closure, or preferably split skin grafting, is the safest approach for the majority of open fractures. However, with low-energy fractures and benign wounds, immediate wound closure can be considered. If primary closure is chosen, the surgeon must watch carefully for signs of wound infection.
If closure is delayed, it should be completed as soon as it is safe to do so, in order to minimize the risk of secondary hospital infection.
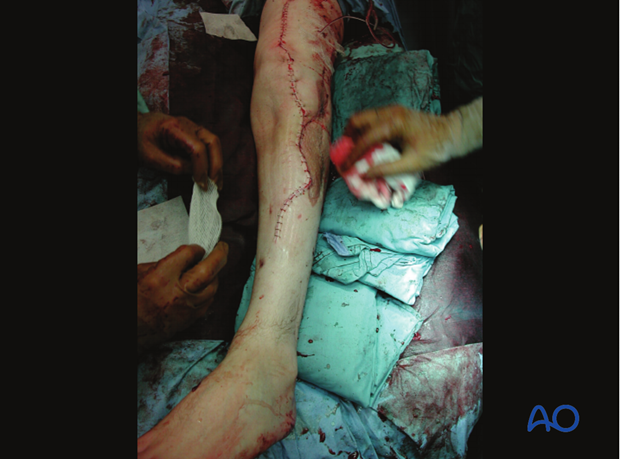
Caveat - Prof. Harald Tscherne 1984
“The most frequent causes of infection in patients with open fractures are:
- incomplete excision of poorly vascularized tissue, especially muscle, skin and bone
- inadequate hemostasis and hematoma evacuation, and insufficient drainage of wound discharges and wound hematoma
- devascularization of primarily viable tissue
- large metallic fixation devices implanted under poorly vascularized tissue
- wound closure under tension
- a failure to recognise compartment syndrome.”

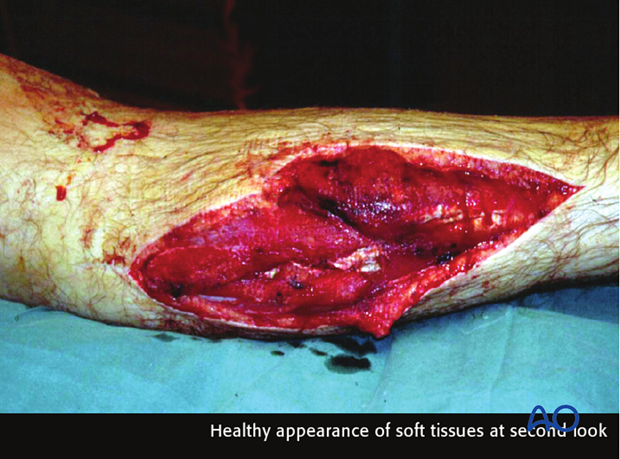
Second look
Forty-eight hours after the original débridement, it is generally advisable to reinspect the injury zone under anesthesia – so-called “second look”.
This affords the opportunity:
- To assess the viability of the soft tissues
- To conduct any necessary further tissue excision
- To wash out any accumulated blood clot, tissue fluid coagulum or remaining foreign material
6. Primary amputation
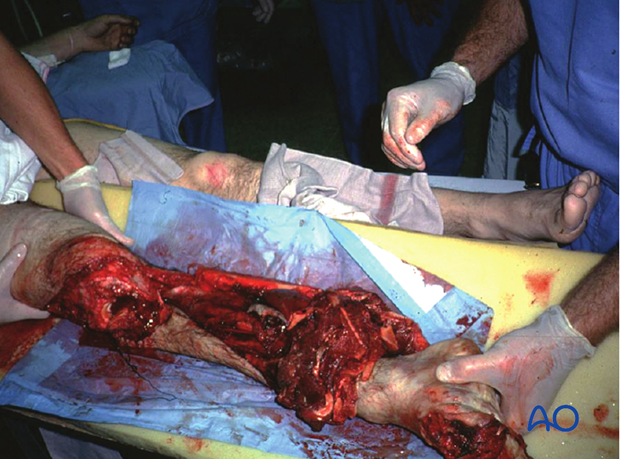
Primary amputation for open fractures
A mangled extremity is a life-threatening injury. Some extremity injuries are so severe that amputation is a safer and more humane option than attempted limb preservation. Injudicious efforts at salvage may be doomed to failure, with the risk of life-threatening complications, particularly infection.
The decision whether to amputate, or to try to save, a severely injured limb is one of the most controversial in trauma surgery. The patient’s physical (and emotional) ability to tolerate injury and prolonged, extensive treatment must be taken into account.
The level of surgical resources at the disposal of the treating surgeon will also influence the choice.
Limb salvage usually requires multiple operations, prolonged hospitalizations, and frequently results in serious complications, ending with a painful and dysfunctional extremity. It was often said by Prof. Sig Hansen that after 2 years of such a program, if the limb was not definitively rehabilitated, the patient would be likely to be “depressed, drug addicted, destitute and divorced!”
Whenever possible, options and outcomes must be discussed with the patient and/or family at an early stage, either before amputation, or before starting out on a long and complex journey of reconstruction.
Because prostheses are generally more functional replacements for the lower than the upper limbs, additional risks may be worth considering to save a severely injured arm, particularly in well-resourced health care systems.
Appropriate primary amputation usually results in a wound which heals satisfactorily, effectively preventing infection, and early return to function: it can often be a kindness to the patient.

Even in constrained health economies, relatively simple lower limb prostheses can be manufactured locally.
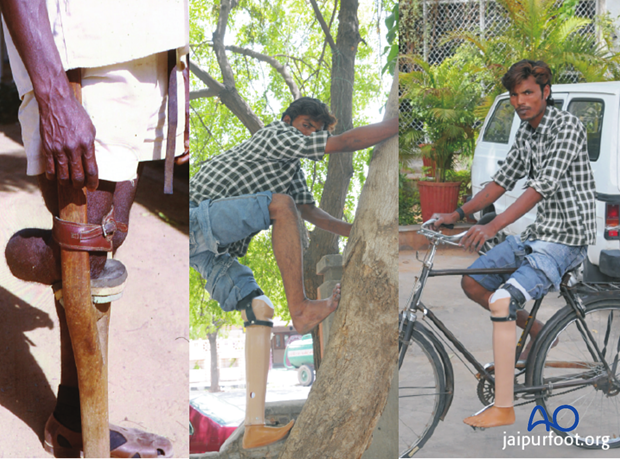
7. Modifiable risk factors
Poor nutrition
In the malnourished, dietary supplements, vitamins and other forms of nutritional support should be instituted as soon as possible after emergency surgery.
Malnourished patients have difficulty healing wounds and resisting infection. Simple screening tests, such as total lymphocyte count (<1.2 x 109 / L), or serum albumen level (<3.4 - 5.4 g/dL), together with a careful dietary history and physical examination, help to identify patients with inadequate nourishment. Severe malnutrition should be corrected as soon as possible after the emergency surgery.
Temperature control
Should a patient’s core temperature fall during surgery, the risk of delay of soft-tissue healing, and of infection becomes greater. For this reason, every effort must be made to minimize intraoperative heat loss, using appropriate covers and external warming devices.